Systemic lupus erythematosus (SLE) is a typical systemic autoimmune disease that commonly affects women in their 20s and 30s, with a male-to-female ratio of 1:9–1:10.1–5 The disease affects multiple organs in the body, including the skin, joints, kidneys, serosa, lungs, central nervous system and blood vessels, as well as the haematopoietic system, thereby presenting various clinical symptoms. In particular, renal involvement in SLE, known as lupus nephritis, is a major determinant of prognosis. For more than half a century, the treatment of SLE has been based on the use of non-specific drugs such as glucocorticoids and immunosuppressive agents, which often cause side effects such as infections. The 10-year survival rate is reported to be 70–90%.4 This is a serious figure considering the young age of SLE onset. Infections are the most common causes of death. SLE is pathologically characterized by production of autoantibodies by B cells, and B cells have a central role in humoral immunity and autoimmune diseases. Thus, therapies targeting B cells are expected to be effective for the treatment of SLE. However, many biologics targeting B cells, such as the anti-CD20 antibody rituximab and the anti-CD22 antibody epratuzumab, have seemed promising and have shown positive results in some patients, including those with immune cytopenias, neuropsychiatric disease or severe lung disease, despite their off-label uses, but have not been investigated in patients with SLE and lupus nephritis.6–10 Meanwhile, belimumab, a monoclonal antibody against soluble B-cell-activating factor (BAFF), belonging to the tumour necrosis factor family, is the first biologic approved for SLE, and its efficacy for lupus nephritis has also been reported.11–14
Biology of B-cell-activating factor and its role in systemic lupus erythematosus and lupus nephritis
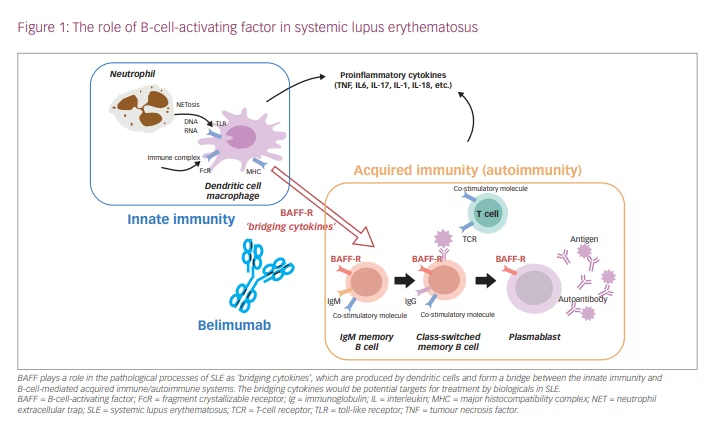
SLE is a multifactorial disease, involving genetic, environmental, immunological, endocrinological and epigenomic control factors.1–5 Genome-wide association studies in SLE have shown that the identified disease susceptibility genes are associated both with the acquired immune system, including T cells and B cells, and with the innate immune system, including intracellular signalling, apoptosis and transcription regulation in dendritic cells.6–9 Genetic and environmental factors induce the production of type I interferon and soluble BAFF from dendritic cells, which leads to disruption of self-tolerance, and induces abnormal activation of T cells and differentiation of B cells into autoantibody-producing cells. Thus, these factors produced by dendritic cells form a bridge between the innate and acquired immune/autoimmune systems. The production of autoantibodies against antigens leads to immune complex formation, which is deposited in the glomerular tissue, activates the complement pathway and causes tissue damage (type III allergy), such as lupus nephritis.6–9 Damage to multiple organs in the body, including the kidneys, occurs through these mechanisms, resulting in a wide variety of clinical manifestations. In fact, patients with SLE and lupus nephritis have an increased expression of interferon-related genes, and increased serum levels of interferon-α and soluble BAFF are observed.7–9
BAFF (also known as B-lymphocyte stimulator [BLyS], CD257) is a type II transmembrane homotrimeric protein of the tumour necrosis factor ligand family with a molecular weight of 18.5 kDa, and is expressed on the cell surface of dendritic cells and macrophages.11–13 It is also released as a soluble BAFF by cutting the extracellular domain. The trimeric protein binds to BAFF receptors on the surface of B cells, and 60-mer BAFF binds to the transmembrane activator and cyclophilin interactor and B-cell maturation antigen, which prevents apoptosis of autoreactive B cells, and induces class switching and differentiation into antibody-producing cells (Figure 1). BAFF-transgenic mice developed SLE-like diseases.15 In patients with SLE and lupus nephritis, an increase in the serum soluble BAFF level was correlated with disease activity, serum anti-double-stranded DNA (dsDNA) antibody titre and organ damage such as nephritis.14,16,17 Given its association with disease progression, soluble BAFF has gained attention as a therapeutic target for SLE.
Treatment strategy of systemic lupus erythematosus
Early treatment is important for the prognosis of SLE; therefore a rapid and accurate diagnosis is essential. The diagnosis of SLE is made based on the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) 2019 classification criteria for SLE.15 Patients with an antinuclear antibody titre of ≥1:80 undergo clinical and immunological examination using the scoring system, and those with a score of ≥10 are diagnosed with SLE. Patients with class III or IV lupus nephritis on renal biopsy according to the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) are also diagnosed with SLE if they have a score of ≥10 according to the abovementioned criteria.18 The sensitivity and specificity of the EULAR/ACR scoring system are superior to conventional diagnostic criteria regardless of race, and its ability to differentiate an SLE diagnosis from infections, malignant tumours and drug-induced disorders is an important advantage.15
In 2014, working groups in Europe and other countries advocated a treat-to-target approach for SLE in clinical practice.19 It was recommended that the treatment target for SLE should be remission of systemic symptoms and organ manifestations. It was stated that the treatment of patients with SLE should aim to optimize quality of life and to ensure long-term survival by minimizing comorbidities and drug toxicity and preventing organ damage. Although the remission criteria were not defined, the use of organ damage indices and systemic disease activity measures such as the SLE Disease Activity Index (SLEDAI)
are recommended.19
Indications and initial doses of glucocorticoids and immunosuppressive agents are determined based on a comprehensive assessment of disease activity, major organ disorders, and complications such as infections and cardiac diseases. Lupus nephritis treatment is selected based on the ISN/RPS histological classification.1,20 Hydroxychloroquine is recommended for all patients, while those with class III or IV lupus nephritis are treated with glucocorticoid pulse therapy followed by high-dose glucocorticoids and immunosuppressive agents (intravenous cyclophosphamide pulse therapy [IV-CY] or mycophenolate mofetil [MMF]). If there is no improvement after 6 months, it is recommended to change the immunosuppressive agent or initiate belimumab therapy. Patients with class V lupus nephritis are treated with medium-dose glucocorticoids, MMF, angiotensin receptor antagonists, and angiotensin-converting enzyme inhibitors, and possibly tacrolimus. Drugs used in maintenance therapy after remission induction include hydroxychloroquine, MMF, azathioprine and tacrolimus, while glucocorticoids should be tapered by 3–6 months after treatment initiation and be discontinued as soon as possible whenever possible.1,19,20
Belimumab therapy in systemic lupus erythematosus
Belimumab is a human immunoglobulin G1 (IgG1) monoclonal antibody that binds to soluble BAFF.13 In the phase III clinical trials of belimumab in subjects with SLE (BLISS), the primary endpoint was defined as the achievement of an SLE responder index 4 (SRI-4) response. SRI-4 consists of scores derived from the SLEDAI, British Isles Lupus Assessment Group (BILAG) index and Physician Global Assessment (PGA).21 SRI-4 response is defined as a ≥4-point reduction in SLEDAI, no new BILAG A or no more than one new BILAG B domain score, and no deterioration from baseline in the PGA by ≥0.3 points.
The BLISS-52 study (ClinicalTrials.gov Identifier: NCT00424476) (a phase III international study of belimumab) investigated 865 patients with SLE with moderate disease activity and a SLEDAI score of ≥6.22 The study reported that the proportion of patients achieving SRI-4 response at 52 weeks was significantly higher in the belimumab group (intravenous infusion of 10 mg/kg at 0, 2 and 4 weeks, and then every 4 weeks) than in the placebo group (58% versus 44%). Significant differences between the two groups were also found in the decrease in autoantibodies, increase in complement activation, number of patients with glucocorticoid dose to ≤7.5 mg (prednisolone equivalent), and improvement in patient’s quality of life and fatigue. In addition, a baseline SLEDAI score of ≥10, anti-dsDNA antibody positivity, and hypocomplementemia contributed to achieving SRI-4 response at 52 weeks. There were no significant differences in the incidence of adverse events, including serious adverse drug reactions and infections.22
The BLISS-76 study investigated 819 patients and reported that the proportion of patients achieving SRI-4 response at 52 weeks was significantly higher in the belimumab 10 mg/kg group than in the placebo group (43% versus 34%).23 Based on these results, belimumab was the first approved biologic for SLE in 2011.
The BLISS-NEA (ClinicalTrials.gov Identifier: NCT01345253) phase III study was conducted in northeast Asia (Japan, Korea and China), and enrolled 705 patients with SLE on standard therapy with autoantibody positivity and a SLEDAI score of ≥8.24 The proportion of patients achieving SRI-4 response at 52 weeks was significantly higher in the belimumab group than in the placebo group (54% versus 40%). In 60 Japanese patients, the figures were 46% versus 25%, respectively. The prolongation of time to relapse and the reduction in glucocorticoid dose were also greater in the belimumab group than in the placebo group.24 However, there were no significant differences in safety between the belimumab and placebo groups.24 The analysis of SLEDAI scores showed a significant improvement in the musculoskeletal, renal and immunological items, a decrease in serum anti-dsDNA antibody titre, an increase in complement activity and a reduction in glucocorticoid dose in the belimumab group compared with the placebo group.25
The BLISS-SC (ClinicalTrials.gov Identifier: NCT01484496) study investigated 836 patients with SLE and a SLEDAI score of ≥8, and reported that the proportion of patients achieving SRI-4 response at 52 weeks was significantly higher in the belimumab group (200 mg subcutaneous injection once weekly) than in the placebo group (61% versus 48%).26 The safety profile was unremarkable.
No notable adverse events were observed in any of the clinical studies. However, a post-marketing surveillance study reported the occurrence of progressive multifocal leukoencephalopathy due to reactivation of John Cunningham virus, depression (0.4%), suicide ideation and suicide attempts, although the causal relationship with belimumab is unknown.26
The long-term extension of randomized trials supports not only a favourable safety profile of belimumab, with a relatively low incidence of serious and opportunistic infections, but also an efficacy with significant reduction in severe flares, lower cumulative exposure to glucocorticoids, lower accrual of irreversible organ damage and improved health-related quality of life.27–29 In the BLISS-NEA study, improvement in the SLEDAI renal items (urinary cast, haematuria, proteinuria and pyuria) was observed by the 7-year follow-up.30 Another study also reported that the addition of belimumab to standard-of-care treatment was effective in reducing glucocorticoids, preventing relapse of disease activity and preventing the progression of organ damage in patients with SLE during maintenance therapy after obtaining remission or low disease activity.31 These findings may be important aspects in the treat-to-target context during maintenance therapy for SLE.
Belimumab therapy in lupus nephritis
From the BLISS-52 and BLISS-76 studies, a subanalysis of 267 patients with lupus nephritis was performed.32 The belimumab 10 mg/kg group showed improvement in urine protein and SLEDAI renal items, decrease in nephropathy relapse, and normalization of complement activity and dsDNA antibody titre at 52 weeks, compared with the placebo group. This tendency was prominent in patients with lupus nephritis with high anti-dsDNA antibody titres and low complement activity. When participants were limited to patients receiving MMF, the belimumab 10 mg/kg group showed a significant improvement at 52 weeks compared with the placebo group, both in urinary protein and complement activity, as well as in SLEDAI score, BILAG score and the proportion of patients achieving SRI-4 response.32 However, interpretation of the results should be done carefully because patients with acute, severe lupus nephritis were excluded from the trials and renal biopsies were not performed in most of patients.
The phase III BLISS-LN (ClinicalTrials.gov Identifier: NCT01639339) placebo-controlled trial included patients with SLE and lupus nephritis.33 Patients had antinuclear antibody positivity or anti-dsDNA antibody positivity, a urinary protein:creatinine ratio of ≥1.0 and ISN/RPS class III–V (including active phase). Patients initially received standard treatment, including high-dose glucocorticoid therapy and MMF or IV-CY for 60 days, and were consequently treated with belimumab 10 mg/kg or placebo. Patients who were refractory to both IV-CY and MMF, and those with an estimated glomerular filtration rate (eGFR) of <30 mL/min/1.73 m2, were excluded from the analysis. The dose of glucocorticoids was reduced to 10 mg during the first 24 weeks of induction therapy, and maintenance therapy was continued for 104 weeks. Primary evaluation was performed at 104 weeks. The mean age of the patients was approximately 33 years. The mean disease duration of lupus nephritis was approximately 0.2 years and the mean SLEDAI-S2K score was approximately 12. Histological examination of the kidney resulted in at least 80% of patients being classified as having class III or IV lupus nephritis.33
The primary endpoint was the primary efficacy renal response (PERR) at 104 weeks, which was defined as a urinary protein:creatinine ratio of ≤0.7, eGFR that was not less than 20% of the value before the renal flare or ≥60 mL/min/1.73 m2, and no use of rescue therapy.33 At 104 weeks, significantly more patients in the belimumab group than in the placebo group had a PERR (43.0% versus 32.3%). The secondary endpoints were complete renal response rate (CRR) at 104 weeks, time to relapse or death, ordinal renal response rate and PERR at 52 weeks. CRR was defined as the percentage of patients who met the following three criteria: a urinary protein:creatinine ratio of ≤0.5, an eGFR that was not less than 10% of the value before the renal flare or ≥90 mL/min/1.73 m2 and no use of rescue therapy. At 104 weeks, significantly more patients in the belimumab group than in the placebo group had a CRR (30.0% versus 19.7%). Significant differences were also found in time to relapse or death at 104 weeks, urinary protein:creatinine ratio, urinary sediment, CRR based on the ordinal renal response rate by eGFR, and PERR at 52 weeks. In addition, the use of MMF at baseline, class III or IV lupus nephritis, and races other than Black contributed to PERR and CRR at 104 weeks. There were also significant differences in SLEDAI-S2K scores (27.8% versus 18.4%), proportion of patients receiving prednisolone ≤7.5 mg or ≤5.0 mg, and positivity rate of anti-dsDNA antibody at 104 weeks. Furthermore, in a secondary analysis of the BLISS-LN study, belimumab could significantly attenuate the risk of lupus nephritis flare and eGFR decline, compared with standard of care and placebo, in patients with lupus nephritis.34 There were no significant differences between the belimumab and placebo groups in the incidence of adverse events, including serious events and events necessitating drug cessation.
Thus, the BLISS-LN study indicated that the addition of belimumab to standard-of-care treatment appeared to be effective in preserving long-term kidney function in patients with lupus nephritis. However, there are several limitations to this study, and questions remain. First, the study outcomes were set for nephritis, but SLE is often complicated with multiple organ manifestations, and mean SLEDAI-2K scores were 12.5. The effects of belimumab on other organs and changes in composite disease activity such as SRI-4 and the BILAG-based Composite Lupus Assessment (BICLA) would be interesting. Second, in the BLISS-52 and BLISS-76 studies, SLEDAI score ≥10, anti-dsDNA antibody positivity and hypocomplementemia at baseline contributed to the achievement of the SRI-4 response at 52 weeks.35,36 Determining baseline factors that are predictive of responsiveness to belimumab in lupus nephritis would be useful after it is approved for the disease. Third, the prevalence and severity of lupus nephritis are known to be more severe in Black and Asian patients than in Caucasian patients, but the number of enrolled patients with different ethnicity was limited in the trial, warranting further study to demonstrate and clarify racial differences.37,38 Fourth, the concomitant immunosuppressants were limited to cyclophosphamide or MMF. However, azathioprine and calcineurin inhibitors such as tacrolimus are often used for lupus nephritis; therefore further study will be needed. Finally, the effectiveness of combination therapy of rituximab and belimumab in lupus nephritis requires further investigation. The upregulation of serum BAFF levels after rituximab therapy is often observed and is known to promote re-expansion of autoreactive B cells, which results in lower frequencies of clinical responses to rituximab.39
In the CALIBRATE study (ClinicalTrials.gov Identifier: NCT02260934), sequential administration of rituximab and belimumab was performed in patients with lupus nephritis.40 Although the trial did not find any significant clinical benefit of the addition of belimumab to standard-of-care treatment, more sustained B-cell depletion, diminished maturation of transitional to naïve B cells and enhanced negative selection of autoreactive B cells were observed.40 Larger studies investigating the addition of belimumab to standard-of-care treatment are warranted.
Conclusions
The most common cause of death in patients with SLE, including lupus nephritis, is infection, which is mainly due to immunocompromised conditions caused by treatments such as glucocorticoids and immunosuppressive agents. A treat-to-target approach for SLE has been proposed.19 Clinical studies have started to evaluate the clinical outcomes, including glucocorticoid dose reduction and cessation, and an interventional study without using glucocorticoids is also ongoing.39 Genome-wide association studies in SLE have shown that the identified disease susceptibility genes are associated not only with B-cell signalling, but also with the innate immune system, including intracellular signalling in dendritic cells.5,6 An international phase III study showed that belimumab, a monoclonal antibody against soluble BAFF, was effective in patients with SLE and those with lupus nephritis; therefore, future indications may include earlier use of belimumab for SLE and lupus nephritis.22,23,33 In addition, anifrolumab, an anti-interferon-α receptor monoclonal antibody, has been approved in the USA and Japan.42,43 Both BAFF and interferon α are produced by dendritic cells, which induce differentiation of B cells. ‘Bridging cytokines’ that connect the innate and acquired immune systems have been receiving attention for the development of new molecular-targeted drugs and are expected to contribute to the establishment of new therapeutic approaches.4