Systemic lupus erythematosus (SLE) is a multisystem, autoimmune disorder affecting skin, joints, kidneys, the heart, lungs, the brain and blood cells, and is characterized by heterogeneous presentations and severity. It is more common in females than males, with a ratio of 9:1, and it is associated with significant morbidity and mortality.1 Despite recent novel therapies being licensed for the management of SLE,2 there is still an unmet need for therapeutic agents with low toxicity that can control the disease and prevent recurrence, particularly for refractory cases.3
The therapeutic challenges relating to SLE can be attributed to the disease’s complex pathways and the interplay between the factors involved in its development.4 Genetics is perhaps the starting point in SLE development, with multiple modes of contribution. Although single gene defects can be implicated in SLE,5,6 they may not necessarily cause the disease on their own. Over 100 gene loci with polymorphisms have been identified7 and are thought to be associated with SLE development, antinuclear antibody and anti-double-stranded (ds) DNA formation, T cell activation and regulation, and interferon production.8–10
A major driver of SLE development is the loss of tolerance of various components of the adaptive immune system and the subsequent formation of B cell clones producing target autoantibodies, the most notable being anti-dsDNA antibodies in increased quantities.11,12 And a key step is believed to be the aberrant apoptosis, cell clearing and pathologic T helper and T cytotoxic lymphocyte stimulation, with reduced T regulatory function.13,14 Aberrant apoptosis exposes the immune system to various intranuclear components and gives rise to the various antinuclear antibodies commonly associated with SLE and SLE-like illnesses.15
Aside from genetic factors, hormones, smoking, ultraviolet exposure and viruses also contribute to the complex pathophysiology of SLE.16–20 For example, oestrogen upregulates T helper cytokines that activate B cells.21,22 It also acts on increasing expression of B cell activating factor (BAFF and Blys) and interferon signature genes, thus increasing inflammation. While viral antigens such as Epstein–Barr virus can mimic the molecular structure of common SLE antigens; patients with SLE have been shown to have a dysregulated immune response against Epstein–Barr virus.23
B cell depletion in systemic lupus erythematosus
Rituximab, a chimeric monoclonal antibody targeting CD20+ B lymphocytes, was approved in 1997 for the treatment of low-grade B cell non-Hodgkin’s lymphoma.24 The role played by B cells in the pathogenesis of SLE had been recognized by then.25,26 In individuals with SLE, B lymphocytes not only produce autoantibodies, they have also been shown to have various altered characteristics compared with the normal population.27 For example, B lymphocytes in individuals with SLE display an uneven increased amount of class‐switched memory B cells compared with naïve B cells. There is also an exaggerated B cell receptor response with a lower activation threshold, allowing autoreactive B cells to thrive with minimal antigen contact. As such, the potential role of B lymphocyte depletion therapy in autoimmune diseases such as SLE was considered.28 In 2002, Leandro et al.29 treated six female patients with active SLE resistant to standard immunosuppressive therapy on an open-label basis with two 500 mg infusions of rituximab, cyclophosphamide, and high-dose oral corticosteroids. The success of this approach supported the new understanding that, although T cytotoxic and T helper cells play a major role in the development and progression of the autoimmune process, B lymphocytes play an equally clinically significant role in the production of autoantibodies that perpetuate the autoimmune cascade.
The 2008 European Alliance of Associations for Rheumatology guidelines for the management of SLE recommended the use of rituximab for patients with lupus nephritis who are resistant to cyclophosphamide therapy.30 This recommendation is based on small, non-controlled trials with a short follow-up suggesting that up to 50% of patients with SLE refractory to cyclophosphamide for lupus nephritis may have a clinically significant response to rituximab.31,32 Over multiple iterations of subsequent society guidelines, B cell depletion therapy (such as rituximab) was established as a therapeutic option for patients with autoimmune diseases (including SLE), in particular in patients with SLE who did not respond to or could not tolerate other established therapies (such as cyclophosphamide or mycophenolate mofetil).33 This recommendation was also included in the latest guidelines published by the European Alliance of Associations for Rheumatology in 202034 and the British Society for Rheumatology in 2018.35
Expanding upon the role of B cells in SLE, new molecular targets such as B cell growth factors, including B-cell activating factor (also known as B-lymphocyte stimulator), were identified and targeted,36 leading to the development and licensing of belimumab for the treatment of SLE.
Other B cell-targeting therapies have been studied. These include the anti-CD19 monoclonal antibody, obexelimab,37 and the anti-CD22 monoclonal antibody, epratuzumab.38 Therapies targeting additional activation factors were also tried: a proliferating-inducing ligand, atacicept39; inhibitors of B cell intracellular activation pathways, (for example, pathways involving Bruton’s tyrosine kinase), such as fenebrutinib40; and B cell proteasome inhibitors, such as bortezomib.41 These treatments were unsuccessful due to either not meeting their endpoints or their safety profile and adverse events.42 The only new drug to be licensed for the treatment of SLE since belimumab has been anifrolumab, a human monoclonal antibody targeting type I interferon receptor subunit 1.43,44
Outcomes in patients with SLE remain poor, with significant mortality and morbidity.45–47 Some cases remain difficult to manage, and a significant portion of the patient population continues to exhibit variable treatment responses. Furthermore, those showing favourable initial outcomes almost always require on-going maintenance therapy after induction to prevent disease relapse. New therapies are needed for treating resistant SLE. This review explores an exciting emerging therapy for B cell depletion: chimeric antigen receptor (CAR) T cell therapy.
What is chimeric antigen receptor T cell therapy?
CAR T cell therapy is a treatment that has been established for the management of several haematological malignancies, including B cell lymphomas and multiple myeloma, with research looking at implementing this safely and effectively in solid organ tumours.48–51 The principle behind CAR T cell therapy is using the body’s own immune system, which in this case are the CD4+ T helper and CD8+ T cytotoxic lymphocytes, to perform specific programmable therapeutic functions.
The concept of CAR T cell therapy was first described in 1989 by Gross et al.52 CARs were an attempt to utilize and direct the specificity of T cells, in a non-major histocompatibility complex (MHC)-restricted manner. This process provides the T cell with an antibody-like specificity as well as the ability to transmit effectively the signal for T cell activation and execution of its effector functions. At the same time, it allows the T cells to bypass cellular processing and presentation, which would normally be done via antigen-presenting cells expressing MHC class II (in the case of CD4+ T cells) or MHC class I restriction (in the case of CD8+ T cells).
In its current form, CAR T cell therapy is a complex process. The principles and methods behind CAR T cell therapy administration vastly differ from conventional pharmacological therapeutics or receptor targeting therapies via monoclonals such as rituximab. This leads to a unique array of challenges, treatment side effects and potentially different long-term outcomes.
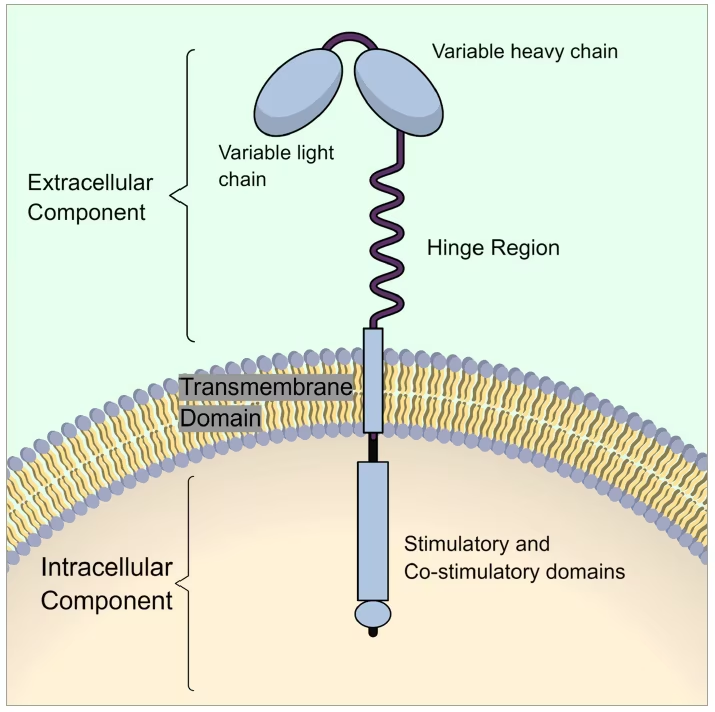
CAR T cell therapy begins with a process called leukapheresis, in which the patient’s white blood cells are harvested.48 The population of CD4+ and CD8+ T lymphocytes is then isolated from the harvested cells using cell processors or centrifugation-based cell separators. Then, a virus carrying genetic material with the instructions for producing CARs, such as a retrovirus or lentivirus, is introduced and transfects the extracted T lymphocytes. The viral genetic material carries the instruction for the formation and expression of the CARs on the surface of the T cells. These CARs that will be expressed on the surface of the T cells are not a naturally occurring receptor. They are a fusion protein of a selected single-chain fragment of the variable (binding) region from a specific monoclonal antibody and one or more T cell receptor intracellular signaling domains, hence the name “chimeric”, i.e., having a part of a B cell receptor/antibody domain in the extracellular part and then connected via a transmembrane domain to a T cell stimulator domain in the intracellular part (Figure 1).
Figure 1:The structure of a chimeric antigen receptor
Once the T cells have been transfected with the instructions for the CARs and the CARs have been expressed on the T cell surface, they undergo a process of stimulation and expansion to increase the number of T cells.53,54 Over 7–12 days, the cells are multiplied in number to achieve clinically significant amounts of CAR T cells. Among the approved CAR T cell therapies, tisagenlecleucel (KYMRIAH®; Novartis, Basel, Switzerland) can have a dose of between 1.2×106 to 6×108 cells per kg of body weight55 and ciltacabtagene autoleucel (CARVYKTI®; Janssen Biotech, Inc. Horsham, PA, USA) a dose of 0.5–1.0×106 cells per kg of body weight, with a maximum dose of 1×108 CAR-positive viable T cells per single infusion.56 After expansion, the CAR T cells undergo purification. The purification process removes other cells that are still in the preparation, as well as different proteins and substances that were used in the previous preparation steps. Finally, the CAR T cells are then re-infused back into the patient (Figure 2).
Figure 2:Steps involved in CAR T cell therapy preparation and administration
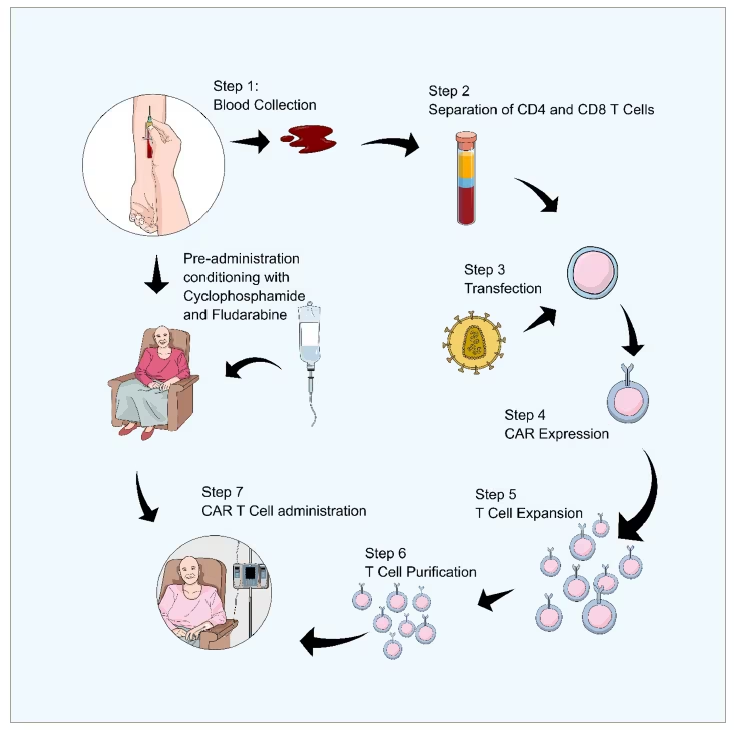
Pre-administration conditioning is a separate process that happens at the same time as steps 1–7.Steps 1–7 are the extraction and preparation of the cells and then the administration back into the patient. As this is happening they also get the pre-adminstration conditioning of cyclophosphamide and fludarabine.
CAR = chimeric antigen receptor; CD = cluster differentiation.
Prior to infusion, the patient must be conditioned and treated with lymphodepleting agents, such as cyclophosphamide or fludarabine. This aims to minimize the profound immunological response that occurs once the CAR T cells are infused, and the patient’s immune system is recruited by the CAR T cells against the tumour or the target antigen. The process from leukapheresis to the administration of the CAR T cells to the patient may take between 14 and 28 days.
The CAR T cell therapy products approved by the United States Food and Drug Administration57 and the European Medicines Agency58 as of March 2023 are detailed in Table 1.
Table 1:Therapies approved by the United States Food and Drug Administration and European Medicines Agency as of 13 March 2023
|
Year first approved by the FDA |
Year first approved by the EMA |
Brand name |
Manufacturer |
Generic name |
Target antigen |
Indication |
|
2017 |
2018 |
KYMRIAH® |
Novartis, Basel, Switzerland |
tisagenlecleucel |
CD19 |
B cell lymphomas |
|
2017 |
2018 |
YESCARTA® |
Kite Pharma Inc., Los Angeles, CA, USA |
axicabtagene ciloleucel |
CD19 |
B cell lymphomas |
|
2020 |
2020 |
TECARTUS® |
Kite Pharma Inc., Los Angeles, CA, USA |
brexucabtagene autoleucel |
CD19 |
B cell lymphomas |
|
2021 |
2021 |
ABECMA® |
Bristol Myers Squibb, New York, NY, USA |
idecabtagene vicleucel |
BCMA |
Multiple myeloma |
|
2021 |
2022 |
BREYANZI® |
Juno Therapeutics, Inc., Seattle, WA, USA |
lisocabtagene maraleucel |
CD19 |
B cell lymphomas |
|
2022 |
2022 |
CARVYKTI® |
Janssen Biotech, Inc. Horsham, PA, USA |
ciltacabtagene autoleucel |
BCMA |
Multiple myeloma |
BCMA = B-cell maturation antigen ;CD = cluster differentiation;EMA = European Medicines Agency;FDA = Food and Drug Administration.
In the United Kingdom, the National Health Service and the National Institute for Health and Care Excellence currently recommend axicabtagene, tisagenlecleucel59 and brexucabtagene autoleucel60 for the treatment of B cell lymphomas.
Chimeric antigen receptor T cell therapy, a new concept in B cell depletion in the treatment of systemic lupus erythematosus
The potential for applying CAR T cell therapy to the treatment of SLE was first recognized in 2019 by Kansal et al.,61 who showed its feasibility and efficacy in mice models. They demonstrated that, by injecting CAR T cells to target CD19+ B cells in mice with SLE, the mice were able to live to the age of 18 months. This outcome differed significantly from that of mice that did not receive the treatment and had to be euthanized for advanced disease. This study also showed improvements in proteinuria, splenomegaly and reduction in anti-dsDNA antibody levels. These findings were supported by a further murine model62 and by the case of a 41-year-old female with a 20-year history of SLE who developed stage IV diffuse large B cell lymphoma and was treated with CAR T cell therapy against CD19.63 Following treatment, the woman from the case study achieved remission, which was confirmed by bone marrow aspirate and flow cytometry. The patient’s B cells remained at undetectable levels until Day 198, with recovery to normal levels at 9 months post therapy. Despite discontinuing prednisone and immunosuppression, the patient’s C3 and C4 complement levels remained within normal limits. Various antinuclear antibody titres were highly elevated prior to therapy; however, nuclear, cytoplasmic and granular antinuclear antibodies were undetectable for up to 37 weeks. The remission of both the lymphoma and SLE was seen up to 23 months following the administration of the CAR T cell therapy.
In 2021, the New England Journal of Medicine published a case report of a patient with SLE treated with CAR T cell therapy.64 The patient had World Health Organization class IIIA lupus nephritis (i.e. focal proliferative disease with active lesions), pericarditis, pleurisy, rash, arthritis and a history of Libman–Sacks endocarditis. The patient had previously been treated with hydroxychloroquine, high-dose glucocorticoids, cyclophosphamide, mycophenolate mofetil, tacrolimus, and the B cell-targeting therapies belimumab and rituximab. Despite these treatments, the disease was not controlled; consequently, the patient was treated with CAR T cell therapy targeting CD19+ B cells. Within 5 weeks of receiving the infusion, the patient achieved clinical and biochemical remission. At 44 days post infusion, the patient remained in remission.
In November 2022, Nature Medicine published a report by Mackensen et al.,65 which detailed the treatment of five cases of refractory SLE with CAR T cell therapy. The report describes how these patients had failed multiple prior treatments: pulsed glucocorticoids (5/5), hydroxychloroquine (5/5), mycophenolate mofetil (5/5), belimumab (5/5), cyclophosphamide (3/5) and azathioprine (2/5). Only one of the patients had previously received rituximab.
Both studies reported clinical improvement along with normalization of complement levels, the disappearance of anti-dsDNA antibodies and the resolution of proteinuria. Both studies also found that all patients achieved remission with CAR T cell therapy without needing further immunosuppressive therapy after several months of follow-up. In the five patient case series, the drug-free remission continued in the follow-up period, despite B cell reconstitution, which occurred on average after 110 ± 32 days. The follow-up periods in the case series were 17, 12, 8, 7 and 5 months.
These studies are the first therapeutic attempts at applying CAR T cell therapy in SLE in humans and provide an impressive and remarkable result. The treatment was offered under compassionate use, and it could be postulated that the patients would have experienced significant morbidity and perhaps even mortality without this intervention.
CAR T cell therapy advantages over established therapies
In the context of SLE treatment, CAR T cell therapy is in essence a B cell depleting therapy similar to rituximab. In reality, there are several stark differences.
The first difference is due to the very nature of rituximab being a chimeric protein and its impact on its binding to CD20 receptors.66 The other is the reduced ability of rituximab to target tissue B cells.67 This means that some B cells evade depletion when CD20 is targeted with rituximab.
Thirdly, rituximab targets CD20, while CAR T cell therapy targets CD19. Although many B cells express both antigens, mature B cells and plasma cells start to lose CD20 expression but continue to express CD19 while migrating into tissues.68 This suggests that CAR T cell therapy targeting CD19+ B cells offers a more significant depletion of B cells and plasma cells and thus a more complete ‘immune reset’.69,70
Finally, rituximab’s half-life is 22 days and is subject to various pharmacokinetic and pharmacodynamic effects that can limit its response and, hence, whether it has a lasting effect.71 Patients may also develop antidrug antibodies against rituximab, limiting repeated use.72 CAR T cells, on the other hand, are living cells that go on to exert a more lasting effect.73 Their persistence and the factors dictating their long-term behaviour can be influenced via alterations to the design of the CARs. For example, including CD28 in the CAR structure appears to generate effector memory cells. Newer CAR designs are created to improve efficacy and limit toxicity while striving to achieve tumour-specific functions.74 How all these advances in the CAR design will directly affect the treatment of patients with SLE remains to be seen.
Side effects and safety
CAR T cell therapy is characterized by a profound immune response led by the T cells. This immune response has been associated with well-described syndromes, namely cytokine release syndrome (CRS), neurotoxicity, cytopenias and hypogammaglobulinaemia.75–79
The flurry of immune cell activation that occurs when the CAR T cells are introduced leads to an abundance of activated immune cells and the overproduction of pro-inflammatory cytokines. This causes symptoms ranging from hypotension and increased transaminases to vascular leak syndrome, acute respiratory distress syndrome and encephalopathy. Patients with CRS were found to have very high levels of interleukin (IL)-6, leading to the approval of tocilizumab for the treatment of post-CAR T infusion CRS.80 CRS is now graded according to severity, with identifiable risk factors predicting the onset of CRS and its severity.81
The latest grading system proposed by the American Society for Transplantation and Cellular Therapy is based on the presence of fever, hypotension and hypoxia.82 Severity ranges from grade 1 to grade 4 depending on fever, the number of vasopressors needed and the amount of oxygen supplementation therapy required.
Neurotoxicity may also occur following CRS, with leakage of various immune cell components crossing the blood–brain barrier.78,79 Patients with neurotoxicity typically experience headaches, confusion, aphasia, dysphasia or somnolence. In addition to IL-6 antagonists, IL-1 antagonists were also found to be effective in the treatment of neurotoxicity.
Overall, nearly 100% of patients receiving CAR T cell therapy against CD19+ B lymphocytes to treat B cell lymphoma develop CRS, with at least 10% developing grade 3 to grade 4 CRS.83 Despite this, a Cochrane Review of 13 studies with a total of 679 participants receiving CAR T cell therapy for relapsed or refractory diffuse large B cell lymphoma found that no deaths were reported due to treatment.84 In the six patients with SLE treated with CAR T cell therapy targeting CD19+ B cells, side effects related to CAR T cell therapy were minimal. Five patients with SLE had no or only mild CRS. Fever (grade 1 CRS) occurred in only three of the six patients, and one patient only required a single infusion of 8 mg per kg body weight tocilizumab, with immediate cessation of the symptoms. None of the patients developed neurotoxicity. An explanation offered by Mackensen et al.65 as to why the patients with SLE receiving CAR T cell therapy had lesser side effects is the lower B cell burden in patients with SLE compared with patients with active B cell malignancies.
CAR T cell therapy targeted against CD19 causes the depletion of CD19+ B lymphocytes. This depletion can lead to hypogammaglobulinaemia and cytopenia.85 There is currently no evidence on how to manage this specifically. Prophylactic immunoglobulins are not currently recommended. Vaccinations are indicated before administering CAR T cell therapy targeting CD19, as with existing B cell-depleting treatments.
During the coronavirus disease 2019 pandemic, patients that received CAR T cell therapy were tested for antibody spike titres against the severe acute respiratory syndrome coronavirus 2. In one study, only 29% of patients were able to mount a clinically relevant antibody response.86 Clinically relevant vaccine responses appeared to improve with repeated vaccine doses.87
Other conditions that have been linked to B cell depletion therapies, such as progressive multifocal leukoencephalopathy, were found to occur in 0.9 cases per 1,000 recipients of CAR T cell therapy targeting CD19.88 This is much less common in patients receiving rituximab.89
Although the six patients with SLE treated with CAR T cell therapy have only experienced relatively minimal side effects to date, larger trials with longer follow-up periods are needed.
Practicality and feasibility
Although CAR T cell therapy usage is increasing, it is associated with several limitations and barriers, especially within the context of severe refractory autoimmune disorders.90,91
First, to administer CAR T cell therapy, the T cells must initially be extracted from the patient to undergo leukapheresis, transfection, expansion and then re-administration into the patient’s bloodstream (Figure 2). The administration itself must be preceded by leuko-ablation or conditioning to prevent CRS and immunotoxicity. The administration period, which is usually up to 14 days, may be too long for some patients with SLE who may require more urgent management.
Second, CAR T cell therapy is dependent on extracting T cells in specific quantities and of a certain quality and purity. However, patients with active SLE may experience alterations to their immune homeostasis or co-existing infections that prevent this. Nonetheless, this risk appears to be theoretical, and recent evidence shows that successful extraction is possible and feasible.92
Finally, the cost may also be a limit to the widespread implementation of CAR T cell therapy in its current form. Currently, the cost of each treatment exceeds £280,000.93 The cost of CAR T cell therapy may potentially become more affordable over the coming years; however, the current cost represents a barrier to accessing this treatment for patients with SLE, not only for compassionate use indications but also for running robust trials to compare it with existing therapies.
The future of CAR T cell therapy
CAR T cell therapy has undergone several improvements in both design and implementation.94 There are now several generations of CAR T cell receptors with various receptor designs and co-stimulatory domains.
Two of the limitations discussed in the previous section, namely the delay in achieving and administering the final product and ensuring the successful extraction of the patient’s T cells, could be overcome by using non-autologous CAR T cells, otherwise known as universal CAR (UCAR) T cells.95 UCAR T cells can be readily administered on demand via a ready-to-use T cell product carrying specific CAR molecules. Using these T cells, therefore, would enable the timely administration of CAR T cell therapy for urgent cases. However, UCAR T cells are not yet available for use in CAR T cell therapy and will need to be designed to overcome both graft versus host disease and graft rejection, among other challenges.
Furthermore, CAR T cell receptors are now being engineered to express different CAR receptors with multiple targets. Alternatively, the CAR T cell product may contain different T cell populations, each with a different CAR receptor.96 This can be applied in oncology for overcoming tumour evasion. However, it remains unclear how this can be applied in autoimmune disorders such as SLE.
Moving away from T cells, research is also underway on using other types of cells, such as CAR natural killer (NK) therapy or even macrophages.97 These alternative therapies would use NK cells or macrophages with engineered CAR receptors to target specific tumour antigens or, potentially, immune receptors associated with autoimmune diseases. NK cells and macrophages exhibit innate antitumour activity and do not induce graft versus host disease. NK cells also have wider immune-activating pathways and cytotoxicity mechanisms, including for solid organs.
Perhaps one of the more ambitious advances in CAR T cell therapy is in vivo CAR generation.98 Rather than extracting the T cells, which involves transfecting and expanding the T cells in vitro and then re-introducing them back into the patient, in vivo CAR generation relies on designing viral vectors that can enter the patient’s blood and lymphatic systems. These vectors both infect and activate naïve T helper and T cytotoxic cells into displaying the specifically engineered CAR receptor. The activated T cells with the new CARs then release and expand in the bloodstream engaging with the target cells expressing the target antigen.
Conclusions
Despite the current challenges associated with CAR T cell therapy, such as its side effects, limited practicality and cost, CAR T cell therapy undeniably represents a promising way of affecting immunomodulation. It has the potential to achieve better control and management not only for refractory SLE cases but also for patients with other autoimmune disorders.
At first glance, CAR T cell therapy in autoimmune disorders appears to follow in the footsteps of rituximab, a haemato-oncological treatment that has crossed over to treat autoimmune disorders. This would be an oversight, as the delivery of CAR T cell therapy is far more complex, the side effect profile is more substantial, and the logistical and financial costs are prohibitive. Unlike with the adoption of rituximab, these factors are likely to be a firmer barrier to widespread adoption. Yet, patients with SLE receiving CAR T cell therapy against CD19 have shown clinical and biochemical improvement, a manageable side effect profile and, most importantly, a drug-free sustainable response. This begs the question of whether this deep reset of the immune system via a more extensive B cell depletion can change the fundamental biology of SLE.
Undoubtedly, the challenges related to CAR T cell therapy will be easier to overcome as research on the current generations of CAR T cell therapy continues to improve and evidence to guide strategies for minimizing side effects and improving safety increases.
We are only beginning to scratch the surface of this technology. CAR T cell therapy is finding uses in an increasing list of conditions, starting with haematological malignancies and moving on to solid tumours and, more recently, to autoimmune disorders. It is rare that a new treatment comes along with so much promise and potential. Further research is needed to reveal whether this exciting new therapy will significantly impact the way we manage SLE.