Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) represents a spectrum of disorders primarily affecting the small blood vessels in various organs. It often affects the kidneys, causing rapidly progressive renal failure due to necrotizing crescentic glomerulonephritis. Another major cause of mortality in AAV is life-threatening pulmonary haemorrhage. Historically, AAV has been divided into granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA) based on the organs affected, the presence or absence of granulomatous inflammation, blood and tissue eosinophilia, history of asthma and the primary target of ANCA antibodies (myeloperoxidase versus proteinase 3).1
In addition to the well-established role of ANCA antibodies, several lines of evidence have emerged, pointing towards the role of the alternative complement pathway of activation in the pathogenesis of AAV. Components of the complement system are not easily detectable at sites of inflammation in AAV and historically their role was considered ancillary at best.1 Therefore, both animal models of AAV and human studies uphold the pivotal role of complement in the development and progression of disease.2–5 Indeed, complement activation and the presence of complement breakdown products tend to correlate with disease severity and outcome.4,6,7 A Swedish biobank study demonstrated that activation of the complement system was an early event associated with MPA.8 The anaphylatoxin C5a, a pro-inflammatory complement activation product, is a potent chemoattractant for neutrophils and monocytes, bringing them to the inflammation sites and causing tissue damage through neutrophil degranulation and release of radical oxygen species and lysosomal proteases.9 This, in turn, can activate the coagulation system and can upregulate the expression of ANCA-autoantigen targets on the surface of activated neutrophils. Studies on mouse models have shown that C5 receptor inhibition can decrease neutrophil activation and their subsequent migration through the vascular endothelium.10,11 Therefore, inhibition of the complement component C5a has emerged as an attractive target for the treatment of AAV (GPA and MPA).
Potential roles for complement inhibitors in the management of ANCA-associated vasculitis
Current treatment protocols for remission induction in patients with severe AAV are based on the use of high-dose glucocorticoids (GCs) combined with either rituximab (RTX) or cyclophosphamide (CYC).12 Unfortunately, a significant percentage of patients with AAV are at risk of developing serious treatment-related side effects or may experience a relapsing course. Novel, better tolerated, less toxic and more effective agents are needed. Several clinical trials, including the CORTAGE trial by the French Vasculitis Study Group, a study by Pepper et al. and, more recently, the Plasma exchange and glucocorticoids for treatment of ANCA-associated vasculitis trial (PEXIVAS), were the first to report that protocols using ≤50% GCs were non-inferior to traditional treatment regimens for induction of remission.13–15 Therefore, there is an urgent need for steroid-sparing therapies in AAV.
Targeted inhibition of complement component C5 has recently emerged as a possible new treatment option, not only in atypical haemolytic uraemic syndrome (HUS), but also in active AAV.16,17 C5 inhibition has been used for treatment of several immune disorders for more than two decades, with proven efficacy. Agents in this group include eculizumab, ravulizumab, avacopan (Tavneos®; ChemoCentryx, San Carlos, CA, USA) and vilobelimab.
Eculizumab works by binding directly to C5, thus preventing its breakdown to C5a and C5b, and avoiding downstream formation of the terminal membrane attack complex.18 Eculizumab was the first US Food and Drug Administration (FDA)-approved C5 inhibitor for treatment of diseases linked to dysregulation of the alternative pathway of complement activation, such as atypical HUS and paroxysmal nocturnal haemoglobinuria. Furthermore, eculizumab successfully reversed acute thrombotic microangiopathy in cases of polymyositis (PM)-scleroderma (Scl) autoantibody-associated scleromyositis and AAV (MPA) complicated with posterior reversible encephalopathy syndrome.19,20 Studies are on-going regarding its potential in treating other secondary thrombotic microangiopathy-associated diseases.18
Another long-acting C5 inhibitor, ravulizumab, has also been studied in atypical HUS,16 but not yet in AAV. However, as both eculizumab and ravulizumab block the activation of the terminal membrane attack complex, severe cases of Neisseria meningitidis infection have been observed in patients treated with these non-selective C5 agents, thus necessitating preventative Neisseria vaccinations before treatment is initiated.16
Avacopan, an oral C5a receptor antagonist, is effective against AAV and was recently approved by the FDA for treatment of patients with AAV, in combination with standard therapy. Inhibiting C5a, as found in the phase III ADVOCATE trial, allowed almost complete avoidance of GCs in patients with AAV.17,21 Data from this trial showed significantly less GC-related toxicity when avacopan was used. Avacopan was not only non-inferior to GC in managing patients with newly diagnosed or relapsing AAV, but also reduced the number of relapses, provided better quality of life and improved glomerular filtration rate. At Week 26, remission was seen in 72.3% of patients receiving avacopan, which was non-inferior compared with 70.1% remission rates in patients given prednisone. At Week 52, avacopan appeared superior even to GC in sustaining AAV remission. Importantly, there were no reports of increased incidence of N. meningitidis infection.
Phase II clinical trials with monoclonal antibody against C5a
Vilobelimab is a newly developed monoclonal antibody against C5a that inhibits neutrophil activation, chemotaxis and complement-driven inflammation/tissue damage.22,23 More than 300 people have already been successfully treated with vilobelimab in several completed clinical trials.22–26 Vilobelimab has been or is currently being explored in a wide variety of conditions including hidradenitis suppurativa, AAV, pyoderma gangrenosum, severe coronavirus (SARS-CoV-2) infection, cutaneous squamous cell carcinoma and sepsis.22–29 Of note, vilobelimab has been shown to efficiently inhibit C5a in patients with severe coronavirus disease 2019 (COVID-19), in a small recent phase II study performed on 30 patients with severe COVID-19 pneumonia.25,30 Another phase IIa clinical trial (SCIENS study) included 72 patients with severe sepsis and septic shock.26 Patients were randomly assigned at a 2:2:2:1 ratio to three dosing protocols for intravenous vilobelimab or placebo, receiving either 2 × 2 mg/kg (at 0 and 12 hours), 2 × 4 mg/kg (at 0 and 24 hours), or 3 × 4 mg/kg (at 0, 24 and 72 hours). While vilobelimab was able to decrease C5a concentration in a dose-dependent manner, membrane attack complex lysis capacity was not affected (as measured by 50% haemolytic complement assay). Vilobelimab was well tolerated without safety issues.26
IXchange study with vilobelimab in
ANCA-associated vasculitis
The recent European-based phase II IXchange study of vilobelimab in AAV (ClinicalTrials.gov Identifier: NCT03895801)28 and the US-based phase II IXPLORE study (ClinicalTrials.gov Identifier: NCT03712345)29 have shown the potential of vilobelimab to reduce the cumulative dose of GCs and GC-related toxicity in patients with AAV. The IXchange study demonstrated a good safety and tolerability profile, according to Peter A Merkel from the Penn Vasculitis Center, UPMC, Pittsburgh, PA, USA, who was the coordinating investigator of the study: “The strong efficacy and safety data in the trial are quite encouraging for the development of this novel agent for the treatment against this organ- and life-threatening disease”.31
The IXchange study was a randomized, placebo-controlled, double-blind, double-dummy, active-controlled, parallel assignment, multicentre, two-part study, which enrolled 57 patients with AAV (GPA and MPA) in total (30 patients were in part 1, and 27 in part 2). Part 1 compared vilobelimab + reduced dose of GC (RDGC) to a standard dose of GC (SDGC), while part 2 compared vilobelimab-only to SDGC. Patients were recruited from various vasculitis clinics throughout Europe. Patients eligible for the study were 18 years or older. Loading doses of vilobelimab were administered on Days 1, 4 and 8, followed by a maintenance dose (800 mg of vilobelimab) every 2 weeks for a total of 16 weeks. This was followed by an 8-week observation period.
The primary outcome of the study was the proportion of patients achieving clinical response (>50% reduction in Birmingham Vasculitis Activation Score [BVAS] compared with baseline and no worsening in any body system at Week 16; efficacy endpoint). Secondary outcomes included:
- safety endpoints at Week 24 (such as treatment-emergent adverse events [TEAE], serious adverse events and adverse events of special interest)
- GC-induced toxicity (measured by the glucocorticoid toxicity index at Week 24)
- pharmacokinetic and pharmacodynamic modelling of vilobelimab treatment at Week 24
- clinical remission, defined as number of patients achieving a BVAS of 0 at Week 16
- vasculitis damage index
- estimated glomerular filtration rate (eGFR).
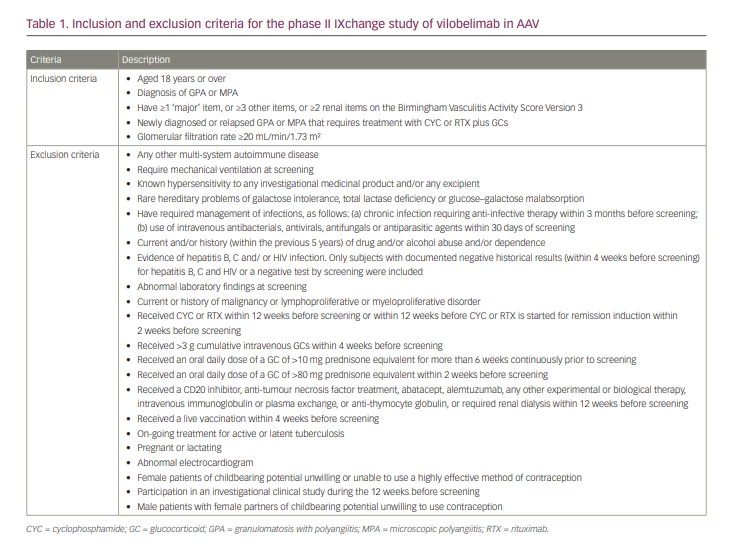
Inclusion and exclusion criteria
Inclusion and exclusion criteria are shown in Table 1. Patients with newly diagnosed or relapsing AAV (GPA or MPA) that required treatment with CYC or RTX plus GCs were included; however, those requiring mechanical ventilation at screening or those with glomerular filtration rate less than 20 mL/min/1.73 m2 were excluded.
Results
Unfortunately, the IXchange study was not statistically powered to demonstrate non-inferiority of vilobelimab compared with SDGC therapy. The primary outcome (% of patients achieving >50% reduction in BVAS at Week 16) was achieved in 16 out of 18 (88.9%) patients in the vilobelimab-only group; in 22 out of 23 (95.7%; one patient missing at Week 16) patients receiving SDGC; and in 10 out of 13 (76.9%; two patients missing at Week 16) patients in the vilobelimab + RDGC group.
The cumulative mean GC dose received during the 28 days prior to randomization was comparable among the three arms, with 1,894.7 mg (vilobelimab only), 1,750.1 mg (SDGC) and 2,438.8 mg (vilobelimab + RDGC). The mean total cumulative dose of GC administered after the screening period until the end of study was 541.9 mg in the vilobelimab-only group, 3,751.3 mg in the SDGC group and 1,485.8 mg in the vilobelimab + RDGC group. As expected, use of vilobelimab instead of GC led to a substantially lower GC composite score. The GC composite score at Week 16 was 0.8 in the vilobelimab-only group, compared with 44.9 in the SDGC group and 26.1 in the vilobelimab + RDGC group. The vasculitis damage index and eGFR were comparable between groups.
The vilobelimab-only group had the lowest number of TEAEs (vilobelimab-only: 81; SDGC: 180; vilobelimab + RDGC: 89). Unfortunately, there was one case of fatal Pneumocystis jirovecii pneumonia (PJP) reported in the vilobelimab-only group, in a patient who did not receive the protocol-recommended PJP prophylaxis. Another non-fatal PJP case was detected in the SDGC group.
The US-based phase II IXPLORE study was a smaller study that enrolled 19 patients.29 Similar to the IXchange study, patients were randomized into three study groups. The IXPLORE study met its primary objective in terms of no safety concerns, but, like the IXCHANGE study, was not powered statistically to show significant efficacy differences. A similar number of TEAEs was observed between the study groups.31
Based on these study results, the drug manufacturer (InflaRx, Munich, Germany) plans to discuss the phase II data with regulatory authorities to determine the next steps in drug development.
Conclusion
In conclusion, a new era in the treatment of AAV based on complement C5a inhibition has already arrived, and in addition to avacopan, other C5-based inhibitors, such as vilobelimab, have a potential to replace GCs altogether in induction protocols for AAV. It is not yet clear whether C5a inhibition-based strategies will eliminate RTX in the maintenance of AAV.