Article highlights
-
Treatment of rheumatoid arthritis remains a challenge, and new interleukin (IL) 6 inhibitors deserve special attention.
-
IL-6 provides pleiotropic effects not only on the pathogenesis of rheumatoid arthritis but also on its comorbidities.
-
The humanized monoclonal antibody olokizumab has a special mode of action by directly suppressing IL-6 (site III).
-
The safety and efficacy of olokizumab were confirmed in large international randomized trials both in biologically naïve patients and in patients with resistance to tumour necrosis factor alpha inhibitors.
Rheumatoid arthritis (RA) is a chronic immunoinflammatory rheumatic disease characterized by the progressive destruction of joints, systemic inflammation of visceral organs and a wide range of comorbidities associated with chronic inflammation.1 The RA pathogenesis is determined by a complex relationship between environmental factors and genetic predisposition leading to an autoimmune response before or in parallel to the development of the clinical symptoms of the disease.2–4 Along with the development of novel drugs, the strategy of RA pharmacotherapy has been improved based on the concepts of ‘treat to target‘5,6 and ‘window of opportunity7 ‘ by diagnosing RA early. This approach determines the possibility of initiating active, tightly controlled anti-inflammatory therapy with disease–modifying antirheumatic drugs (DMARDs), primarily methotrexate and, if necessary, with subsequent biological disease-modifying antirheumatic drugs (bDMARDs).8 However, despite significant progress in the early diagnosis and treatment of RA,9 which led to a clear improvement in the prognosis for many patients, the issues with RA pharmacotherapy are far from resolved.10 This is due to heterogeneous immunopathogenic mechanisms, both at the onset and during the progression of RA, which complicates personalized therapy.
Among the cytokines involved in the pathogenesis of RA and other immunoinflammatory rheumatic diseases, interleukin (IL) 6 is of special clinical value.11-16 The introduction of the monoclonal antibodies (mAbs) tocilizumab first and then sarilumab, which inhibit the proinflammatory effects of IL-6, into clinical practice was considered a great achievement in the treatment of immunoinflammatory rheumatic diseases at the beginning of the 21st century.14
It is worth remembering that IL-6 is a protein consisting of 184 amino acids, with a molecular weight of 26 kDa, two N-glycosylated sites and four cysteine residues. IL–6 was originally described as a B–cell differentiation factor. The biological effects and molecular mechanisms of action of IL-6 are determined by its ability to activate the target genes regulating the differentiation, survival, apoptosis and proliferation of various immune and non-immune cells in the human body. Therefore, IL-6 acts as a pleiotropic autocrine, paracrine and hormone-like regulator of various normal and abnormal biological processes (e.g. development of all forms of acute and chronic inflammation, coordination of innate and acquired immunity, metabolism, neurodegeneration, oncogenesis). The pathogenetic effects of IL-6 and potential effects of IL-6 inhibitors are summarized in Table 117–19 and Table 2.20–40 IL-6 expression and synthesis, predominantly by myeloid cells (e.g. macrophages, dendritic cells), are regulated by various transcription factors, such as nuclear factor kappa beta, which are activated by proinflammatory cytokines (e.g. IL-1β, tumour necrosis factor alpha [TNF–α], IL-17) and pathogen–recognizing Toll-like receptors (Rs), and are controlled by microRNAs, RNA-binding proteins (Roquin, AT-rich interactive domain-containing 5a [Arid5a]), and RNases (Regnase-1), which are all circadian rhythm regulators. The physiological concentration of IL-6 is very low (1–5 pg/mL) but tends to increase rapidly to 1 µg/mL when inflammatory diseases (e.g. RA) or infections (e.g. sepsis, coronavirus disease 2019 [COVID-19]) are present.
Several factors determine the pleiotropic characteristics of IL-6. First, to transmit the intracellular signal, IL-6 binds to the α-chain of the heterodimeric IL-6R Cluster of Differentiation 126 (CD126), with a molecular weight of 80 kDa, forming a complex that then binds to the signal coreceptor, the transmembrane protein gp130 [130 kDa glycoprotein; IL-6Rβ]).41 Second, IL-6Rα is only expressed on the surface of certain cells (i.e. hepatocytes, neutrophils, monocytes, adipocytes, myocytes and some populations of lymphocytes), whereas gp130 is expressed by the vast majority of human cells.42 Therefore, IL-6 shows a high affinity for IL-6Rα and reacts with gp130 only as part of the IL-6–IL-6Rα complex.
The existence of IL-6R in both transmembrane (membrane-bound [m]IL-6R) and soluble forms (sIL-6R) determines the three main forms of IL-6 signalling: classical signalling, trans-signalling and cluster signalling. Classical signalling is mediated by the binding of IL-6 to mIL-6R, whereas trans-signalling is mediated by the formation of the IL-6 complex with sIL-6R, which directly induces the activation of gp130 in cells not expressing mIL-6R. A new IL-6 signalling mechanism, trans-presentation (cluster signalling), has recently been characterized, whereby IL-6 binds to IL-6Rα on the membrane of specific dendritic cells and is presented to gp130 homodimer expressed on the surface of closely spaced T cells.43 This mechanism is believed to play a major role in the implementation of a pathogenic subpopulation of Th17 cells. All IL-6 signalling pathways lead to the activation of the Janus family tyrosine kinase (JAK) pathway, such as signal transducers and activators of transcription 1 (STAT 1) and STAT3, phosphoinositide 3-kinases, mitogen-activated protein kinase and AMP-activated protein kinase, regulating the synthesis of a wide range of biologically active mediators.44 Trans-signalling (and trans-presentation) is believed to be involved in the development of proinflammatory effects of IL-6, whereas classical signalling is largely involved in the physiological regulation of homeostasis and inflammation resolution.
Table 1: Pleiotropic effects of interleukin 6 potentially involved in the pathogenesis of rheumatoid arthritis and concomitant comorbidities17–19
|
Effects |
Role in pathogenesis |
Effect of IL-6 inhibition |
|
Immune Proinflammatory
|
|
|
|
Anti-inflammatory
|
|
|
|
Musculoskeletal Catabolic
Anabolic
|
|
|
|
Haematological
|
|
|
|
Neuronal
|
|
|
|
Cardiovascular and endocrine
|
|
|
BMD = bone mineral density;CRP = C-reactive protein;HbA1c = glycated haemoglobin;IL = interleukin;M2 = alternatively activated macrophage;RANKL = receptor activator of nuclear factor kappa-B ligand;SAA = serum amyloid A;TGF = Transforming growth factor;Th = T helper;TNF = tumour necrosis factor.
Table 2: The main characteristics of interleukin 6 inhibitors20–40*
|
Characteristics |
Tocilizumab |
Sarilumab |
Olokizumab |
|
Molecule |
Humanized IgG1 mAb |
Human mAb |
Humanized IgG4 mAb |
|
Mechanism of action |
Binding to soluble and membrane IL-6R |
Binding to soluble and membrane IL-6R |
Binding to IL-6 site III |
|
Routes of administration |
IV, SC |
SC |
SC |
|
Half-life |
13 days (8 mg/kg) |
10 days (200 mg) |
31 days |
|
Doses |
IV RA – 8 mg/kg every 4 weeks (initial dose 4 mg/kg every 4 weeks) pJIA – 8 or 10 mg/kg every 4 weeks sJIA – 8 or 12 mg/kg every 2 weeks COVID-19 – 8 mg/kg (single dose) GCA – 6 mg/kg every 4 weeks CRS – 8 or 12 mg/kg every 2 weeks SC RA, SSc-ILD and GCA – 162 mg once weekly pJIA – 162 mg every 2 or 3 weeks sJIA – 162 mg once weekly or every 2 weeks |
150 or 200 mg every 2 weeks |
64 mg every 2 or 4 weeks |
|
Formal indications |
RA, pJIA, sJIA, GCA, CRS against CAR-T-cell therapy, COVID-19, SSc-ILD |
RA |
RА, COVID-19 (approved only in Russia) |
|
Main randomized placebo–controlled studies for RA† |
|||
|
OPTION,20 LITHE,21 |
MOBILITY,22 KAKEHASI23 |
CREDO-124 CREDO 225 |
|
TOWARD,26 ROSE,27 SUMMACTA,28 BREVACTA,29 |
|
|
|
RADIATE30 ADACTA‡,31 SATORI,32 AMBITION33 U-ACT-EARLY;34 FUNCTION35
|
TARGET36 |
CREDO 3,37 Genovese et al.,§38 Takeuchi et al.§39 |
|
MONARCH40 |
|
|
|
|||
*Sirukumab is a human monoclonal antibody designed for the treatment of RA. The clinical development programme was terminated by the manufacturer. This product was not approved by the US Food and Drug Administration due to an imbalance in mortality between sirukumab and placebo groups in phase III.
†All trials listed were phase III studies except ADACTA‡ (phase IV) and two phase II studies §(38,39).
CAR-T = chimeric antigen receptor T cell;COVID-19 = coronavirus disease 2019;CRS = cytokine release syndrome;csDMARD = conventional synthetic disease-modifying antirheumatic drugs;GCA = giant cell arteritis;Ig = immunoglobulin;IL = interleukin;IV = intravenous;mAB = monoclonal antibody;p/sJIA = polyarticular/systemic juvenile idiopathic arthritis;R = receptor;RA = rheumatoid arthritis;SC = subcutaneous;SSc-ILD = systemic sclerosis-associated interstitial lung disease;TNF = tumour necrosis factor.
Currently, several bDMARDs specific to IL-6R and IL-6 have been developed. The most well-characterized ones include tocilizumab (Actemra, RoActemra; F. Hoffmann-La Roche Ltd., Basel, Switzerland), which is a humanized mAb targeting IL-6R,45,46 and sarilumab (Kevzara®; Sanofi and Regeneron Pharmaceuticals, Inc., Paris, France), which is a human mAb targeting IL-6R.47,48 The mAbs that block the activity of IL-6 itself include the human mAbs sirukumab,49,50 the humanized mAbs clazakizumab and satralizumab (Enspryng®; F. Hoffmann-La Roche Ltd., Basel, Switzerland),51 and the chimeric mAb siltuximab (Sylvant®; EUSA Pharma, Hemel Hempstead, UK).52
This article focuses on the humanized mAb olokizumab, which binds to IL-6, developed by R-PHARM (Moscow, Russia) under the license agreement with UCB Pharma (Brussels, Belgium).53 The half-life of the product is 31 days, its bioavailability is 65%, and subcutaneous injection volume is 0.4 mL (for dose of 64 mg).
To understand the mechanism of action of olokizumab, it has to be highlighted that IL-6 contains three conservative conformational epitopes: site I, site II and site III (Figure 1). Site I participates in the formation of the IL-6 complex with IL-6R, and site II is a composite epitope interacting with cytokine-binding homologous gp130 site, with the formation of IL-6R–IL-6–gp130 trimeric complex. Subsequent interaction between IL-6 site III with the gp130 immunoglobulin-like activation domain consisting of two IL-6R–IL-6–gp130 trimers leads to the formation of a complete biologically active hexamer signalling complex activating JAK-STAT. Thus, by specifically blocking site III, the mode of action of olokizumab is special, as it limits the ability of IL-6 to form a hexameric signalling complex, thereby suppressing the activation of the JAK-STAT signalling pathway.14,53
Figure 1: Characteristics of interleukin 6 inhibitors
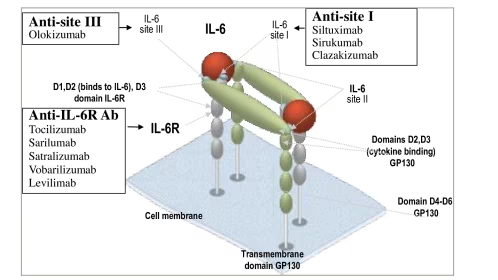
Ab = antibody; IL = interleukin; R = receptor.
Efficacy and safety of olokizumab for rheumatoid arthritis
CREDO 1
The efficacy and safety of olokizumab were investigated in CREDO 1 (ClinicalTrials.gov identifier: NCT02760368), a 24–week multicentre randomized controlled trial (RCT) that enrolled 428 patients randomized 1:1:1 to groups receiving olokizumab 64 mg every 2 weeks, olokizumab 64 mg every 4 weeks or placebo.24 The primary endpoint was achieving the American College of Rheumatology 20% improvement criteria (ACR20) after 12 weeks. Secondary endpoints included the number of patients with Disease Activity Score-C-reactive protein (DAS28-CRP) of <3.2 at week 12, Clinical Disease Activity Index (CDAI) of ≤2.8 at week 24, ACR50 response after 24 weeks and changes in Health Assessment Questionnaire-Disability Index (HAQ-DI) from baseline to week 12.
ACR20 response was achieved in 70.4% of patients treated with olokizumab every 4 weeks, 63.6% of patients receiving olokizumab every 2 weeks and 25.9% of the placebo group (p<0.001) (Table 3).24 Olokizumab had higher efficacy than placebo after 12 weeks, which was maintained for up to 24 weeks. The frequency of DAS28 CRP decrease ≤3.2 was 33.6% with olokizumab every 2 weeks, 38.7% with olokizumab every 4 weeks and 3.5% with placebo (p<0.0001 in both comparisons). Significant improvement in physical function (HAQ-DI) was noted after 12 weeks of treatment with olokizumab compared with placebo (olokizumab every 2 weeks: -0.54; olokizumab every 4 weeks: –0.56; placebo: –0.20; p<0.0001 in both cases). Minimally significant improvement in HAQ-DI (0.22) occurred in 62.2% and 66.2% of patients treated with olokizumab every 2 weeks and every 4 weeks, respectively, compared with 47.6% of patients in the placebo group. The ACR50 response after 24 weeks was reported in 42.7 % of patients receiving olokizumab every 2 weeks, in 48.6% of those receiving olokizumab every 4 weeks and in 7.7% of those receiving placebo (p<0.0001 in both cases). Remission (CDAI ≤2.8) after 24 weeks was achieved in 8.4% of patients treated with olokizumab every 2 weeks, in 7.7% of patients treated with olokizumab every 4 weeks and in no patients in the placebo group (p<0.0003 and p<0.0002, respectively). Olokizumab efficacy (ACR20) was not affected by sex, age, body mass index, initial severity of RA, duration of previous methotrexate therapy, detection of antibodies to cyclic citrullinated proteins and rheumatoid factor. In addition, there was a more pronounced positive change in the Short Form-36 mental and physical domains, the Functional Assessment of Chronic Illness Therapy – Fatigue and other quality–of–life parameters.
Table 3: Efficacy of olokizumab compared with placebo in patients with methotrexate-resistant rheumatoid arthritis (CREDO-1) (12 weeks)
|
Efficacy parameters |
Groups of patients |
||
|
OKZ (every 2 weeks) N=143 |
OKZ (every 4 weeks) N=142 |
Placebo N=143 |
|
|
Primary endpoint |
|||
|
ACR20, n (%) |
91 (63.6) <0.0001 |
100 (70.4) <0.0001 |
37 (25.9) |
|
Secondary endpoints |
|||
|
ACR50, n (%) |
61 (42.7) <0.0001 |
69 (48.6) <0.0001 |
11 (7.7) |
|
DAS28-CRP ≤3.2, n (%) |
48 (33.6) <0.0001 |
55 (38.7) <0.0001 |
5 (3.5) |
|
CDAI ≤2.8, n (%) |
12 (8.4) <0.001 |
11 (7.7) <0.001 |
0 |
|
HAQ-DI, LSM (SE) |
–0.54 (0.04) |
–0.56 (0.04) |
–0.20 (0.04) |
ACR(20/50) = American College of Rheumatology improvement criteria (20%/50% improvement);CDAI = Clinical Disease Activity Index;DAS28-CRP = Disease Activity Score-C-reactive protein;HAQ-DI = Health Assessment Questionnaire-Disability Index;LSM = least squares mean;OKZ = olokizumab;SE = standard error.
Most adverse drug reactions (ADRs) were not severe and occurred in about half of the patients. ADRs leading to discontinuation of treatment were reported in 4.9% of patients who received olokizumab every 2 weeks, 3.5% of patients who received olokizumab every 4 weeks and in 0.7% of patients who received placebo.24 Injection-related reactions were reported in two patients (1.4%) in each olokizumab group. In total, 20 serious ADRs were reported, 5.6% among patients from both olokizumab groups and 2.8% from patients in the placebo group. The most common serious ADRs were infections, which occurred in 2.8% of patients receiving olokizumab every 2 weeks, in 0% of those receiving olokizumab every 4 weeks and of 1.4% in those receiving placebo. The only fatal outcome reported was in a patient receiving olokizumab every 2 weeks and was associated with staphylococcal sepsis resulting in toxic shock. As with treatment with other IL-6 inhibitors, olokizumab was associated with increased lipid levels, although no cardiovascular complications were observed. In very rare cases, moderate thrombocytopenia and neutropenia were reported. Increased serum alanine aminotransferase levels (>3 times the upper limit of normal) were observed in 9.2% of patients treated with olokizumab every 2 weeks, in 11.4% of patients treated with olokizumab every 4 weeks and in 5.0% of patients treated with placebo. Antidrug antibodies were found in 4.4% of patients receiving olokizumab every 2 weeks and in 6.6% of patients receiving olokizumab every 4 weeks. Neutralizing antidrug antibodies were not detected.
CREDO 2
Among the RCTs designed to investigate the efficacy of mAb to IL-6R or IL-6, the CREDO 2 study (ClinicalTrials.gov identifier: NCT02760407) is of particular interest because it was not only placebo controlled but also active comparator controlled (adalimumab) in patients with methotrexate resistance.25 This RCT enrolled 1,648 patients with active RA (a swollen joint count of ≥6 out of 66 joints assessed, a tender joint count of ≥6 out of 68 joints assessed) who met the 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) criteria, with inadequate effect (or intolerance) of methotrexate (≥12 weeks) at a dose of 15–25 mg/week. The patients were randomized (2:2:2:1) to four groups: olokizumab 64 mg every 2 weeks, olokizumab 64 mg every 4 weeks, adalimumab 40 mg every 2 weeks or placebo, in all cases added to methotrexate therapy. The primary endpoint was ACR20 response after 12 weeks. The secondary endpoints were non-inferiority of olokizumab compared with adalimumab with respect to an ACR20 response, reduction of percentage of patients receiving olokizumab with a DAS28-CRP of ≤3.2 compared with both adalimumab and placebo, HAQ-DI changes, ACR50 and CDAI ≤2.8 (remission).
After 12 weeks, the ACR20 response was reported in 70.3% of patients treated with olokizumab every 2 weeks, 71.4% of patients treated with olokizumab every 4 weeks, 66.9% of patients in the adalimumab group and 44.4% of patients in the placebo group (p<0.0001) (Table 4).25 Differences in the efficacy of olokizumab and adalimumab compared with placebo were noticeable after 2 weeks. DAS28-CRP ≤3.2 was achieved in 45.3% of patients treated with olokizumab every 2 weeks, 45.7% of patients treated with olokizumab every 4 weeks, 38.3% of patients treated with adalimumab and 12.8% of patients in the placebo group (all p<0.0001). With olokizumab and adalimumab, the ACR50 response and rate of remission (CDAI ≤2.8) were more frequent compared with placebo.
Table 4: Efficacy of olokizumab compared with adalimumab and placebo in patients with methotrexate-resistant rheumatoid arthritis (CREDO-2)
|
Efficacy parameters |
Groups of patients |
|||
|
OKZ (every 2 weeks) N=464 |
OKZ (every 4 weeks) N=479 |
ADA N=462 |
Placebo N=243 |
|
|
Primary endpoint |
||||
|
ACR20, 12 weeks, n (%) |
326 (70.3) <0.0001 |
342 (71.4) <0.0001 |
309 (66.9) <0.0001 |
108 (44.4) |
|
Secondary endpoints |
||||
|
ACR50, 24 weeks, n (%) |
234 (50.4) <0.0001 |
240 (50.1) <0.0001 |
214 (46.3) |
55 (22.6) |
|
DAS28-CRP ≤3.2, 12 weeks, n (%) |
210 (45.3) <0.0001 |
219 (45.7) <0.0001 |
177 (38.3) <0.0001 |
31 (12.8) |
|
CDAI ≤2.8, 24 weeks, n (%) |
52 (11.2) 0.0008 |
58 (12.1) 0.0003 |
60 (13.0) |
10 (4.1) |
|
HAQ-DI, 12 weeks, LSM (SE) |
–0.64 (0.03) |
–0.61 (0.03) |
–0.61 (0.03) |
–0.42 (0.04) |
ACR(20/50) = American College of Rheumatology improvement criteria (20%/50% improvement);ADA = adalimumab;CDAI = Clinical Disease Activity Index;DAS28-CRP = Disease Activity Score-C-reactive protein;HAQ-DI = Health Assessment Questionnaire-Disability Index;LSM = least squares mean;OKZ = olokizumab;SE = standard error.
In general, ADRs were reported in 68.0% of patients. Infections (upper respiratory tract infection and urinary tract infection) were the most frequent events.25 In most cases, ADRs were mild to moderate and led to discontinuation of treatment in 4.5% of patients treated with olokizumab every 2 weeks, 6.3% of patients treated with olokizumab every 4 weeks, 5.6% of patients treated with adalimumab and 3.7% of patients treated with placebo. The incidence of serious ADRs was 4.8%, 4.2%, 5.6% and 4.9%, respectively. The most common serious ADRs were infections: 1.3% occurred in patients who received olokizumab every 2 weeks, 1.5% in patients who received olokizumab every 4 weeks, 3.5% in patients who received adalimumab and 1.6% in patients who received placebo. ADRs leading to death occurred in three patients (0.6%) treated with olokizumab every 2 weeks, two patients (0.4%) treated with olokizumab every 4 weeks, one (0.2%) treated with adalimumab and one treated with placebo (0.4%). Serious adverse events leading to death were as follows: one case each of stroke, sepsis and septic shock among patients receiving olokizumab every 2 weeks (0.2%); one case each of sepsis and myocardial infarction among patients receiving olokizumab every 4 weeks (0.2%); sepsis in one patient receiving adalimumab (0.2%); and sudden death in one patient receiving placebo (0.4%). Antidrug antibodies were found in 3.8% of patients treated with olokizumab every 2 weeks and in 5.1% of patients treated with olokizumab every 4 weeks. Neutralizing antidrug antibodies were detected in two patients treated with olokizumab every 4 weeks, one of whom lacked a treatment ACR20 response.
CREDO 3
The RCT CREDO-3 (ClinicalTrials.gov identifier: NCT02760433) was designed to evaluate the efficacy and safety of olokizumab in patients resistant to TNF-α inhibitors.37 The study enrolled 368 patients, who were randomized (2:2:1) to three groups: olokizumab 64 mg every 2 weeks, olokizumab 64 mg every 4 weeks and placebo. After 16 weeks, the patients receiving placebo were re-randomized into groups receiving olokizumab 64 mg every 2 weeks and olokizumab 64 mg every 4 weeks. Patients had active RA (a swollen joint count of ≥6 out of 66 joints considerd; a tender joint count of ≥6 out of 68 joints considered), met the ACR/EULAR criteria (2010), had received methotrexate 15–25 mg/week for ≥12 weeks prior to screening and had an inadequate response to at least one anti-TNF agent after ≥12 weeks of treatment. The primary endpoint was ACR20 response after 12 weeks. Secondary endpoints included the number of patients achieving a decrease in DAS28-CRP ≤2.8 after 12 weeks.
The primary efficacy endpoint (ACR20) after 12 weeks was reported in 60.9% of patients receiving olokizumab every 2 weeks, 59.6% of patients receiving olokizumab every 4 weeks and in 40.6% of patients receiving placebo (p<0.01 for both comparisons) (Table 5).37 The difference in therapeutic efficacy between the patients receiving olokizumab or placebo was already observed after 2 weeks and persisted for 24 weeks. Differences were also reported between patients receiving olokizumab every 2 weeks, olokizumab every 4 weeks and placebo according to DAS28-CRP ≤3.2 (secondary endpoint) (p<0.0001 and p<0.0035, respectively). Despite the tendency for more pronounced positive changes in HAQ-DI between the patients receiving olokizumab and placebo, these differences were not statistically significant (Table 5). As in CREDO 1, the efficacy of olokizumab (ACR20) was not affected by sex, age, body mass index, initial severity of RA, duration of previous methotrexate therapy, detection of antibodies to cyclic citrullinated proteins and rheumatoid factor. During the re-randomization of patients treated with placebo to the olokizumab groups after 16 weeks, rapid positive changes were reported in all tested endpoints, reflecting therapeutic efficacy. In addition, positive changes in quality of life (mental and physical domains of the Short Form-36 index) were observed in the olokizumab groups.
Table 5: Efficacy of olokizumab compared with placebo in patients with tumour necrosis factor alpha inhibitor-resistant rheumatoid arthritis (12 weeks) (CREDO-3)
|
Efficacy parameters |
Groups of patients |
||
|
OKZ (every 2 weeks) N=138 |
OKZ (every 4 weeks) N=161 |
Placebo N=69 |
|
|
Primary endpoint |
|||
|
ACR20, n (%) |
84 (60.9) <0.01 |
96 (59.6) <0.01 |
28 (40.6) |
|
Secondary endpoints |
|||
|
ACR50, n (%) |
46 (33.3) <0.01 |
52 (32.3) <0.01 |
11 (15.9) |
|
DAS28-CRP ≤3.2, n (%) |
55 (39.9) <0.001 |
45 (28.0) <0.01 |
8 (11.6) |
|
CDAI ≤2.8, n (%) |
9 (6.5) <0.001 |
5 (3.1) <0.001 |
0 |
|
HAQ-DI, LSM (SE) |
–0.49 (0.05) ≤0.025 |
–0.39 (0.04) |
–0.32 (0.07) |
ACR(20/50) = American College of Rheumatology improvement criteria (20%/50% improvement);CDAI = Clinical Disease Activity Index;DAS28-CRP = Disease Activity Score-C-reactive protein;HAQ-DI = Health Assessment Questionnaire-Disability Index;LSM = least squares mean;OKZ = olokizumab;SE = standard error.
The overall incidence up to 24 weeks of treatmen- emergent adverse events (TEAEs) was 64.7% in particular 64.3% of patients in the olokizumab every 2 weeks group, 59.7% of patients in the olokizumab every 4 weeks group and 50.7% of patients in the placebo group.37 Most TEAEs were mild, and infectious complications were the most frequent. Serious TEAEs were reported in 7.0% of patients treated with olokizumab every 2 weeks, 3.2% patients treated with olokizumab every 4 weeks and no patients in the placebo group. Increased (>3 the upper limit of normal) alanine transaminase levels were observed in 8.7% of patients receiving olokizumab every 2 weeks, 10.0% of patients receiving olokizumab every 4 weeks and 0% of patients receiving placebo. Non-neutralizing antidrug antibodies were found in 6.9% of patients; there was no association between antidrug antibody detection, efficacy of therapy and development of ADRs.
Discussion
The results of these three large-scale, international phase III randomized, placebo-controlled double-blind studies of olokizumab in RA – CREDO 1,24 CREDO 225 and CREDO 337 – have confirmed the efficacy and safety of IL-6 inhibition and have led to approval by the US Food and Drug Administration.54 It is currently unclear whether the biological and clinical effects of mAbs inhibiting IL-6R or IL-6 itself are different. For example, the administration of mAb to IL-6R while retaining IL-6 in the bloodstream leads to the increase of IL-6 serum concentrations. For mAbs to IL-6 (e.g. olokizumab) induction of IL–6 expression has not been observed so far.55 According to the study results, no clear difference was evident between olokizumab and the other IL-6R antagonists for efficacy (Table 6) and safety outcomes (Table 7).20,22,24,25,30,36,37,56–58
Table 6: Comparative efficacy of interleukin 6 inhibitor therapy in rheumatoid arthritis
|
Product (trial name) |
Duration, weeks |
Groups of patients |
Efficacy, % |
||||
|
ACR20 |
ACR50 |
ACR70 |
DAS28-CRP <2.6 |
CDAI ≤2.8 |
|||
|
Resistance to methotrexate |
|||||||
|
Tocilizumab (OPTION†, phase III)20 |
24 |
TCZ 8 mg/kg 4 weeks + MTX (n=205) TCZ 4 mg/kg 4 weeks + MTX (n=213) Placebo + MTX (n=204) |
58.5* 47.8 26.5 |
43.9* 31.4 10.8 |
21.9* 12.2 1.9 |
27.4* 13.4 0.8 |
– |
|
Sarilumab (MOBILITY, phase III)22 |
52 |
SAR 200 mg 2 weeks + MTX (n=399) SAR 150 mg 2 weeks + MTX (n=400) Placebo + MTX(n=398) |
66.4* 58.0 33.4 |
46.0 37.0 17.0 |
12.8* 14.8 3.0 |
34.1* 27.8 10.1 |
13.8* 10.3 5.0 |
|
Levilimab (AURORA, phase II)56 |
12 |
LVM 162 mg + МТX1 week (n=35) LVM 162 mg + МТX2 weeks (n=35) Placebo + MTX (n=35) |
77.1* 57.1 17.1 |
51.4* 31.4 5.7 |
28.6* 20.0 2.9 |
11.4* 5.7 2.9 |
Only change of overall CDAI |
|
Olokizumab CREDO-1 (phase III)24 |
24 |
OKZ 64 mg 2 weeks + MTX (п=143) OKZ 64 mg 4 weeks + MTX (п=142) Placebo + MTX (n=143) |
63.6 70.4 25.9 |
42.7 48.6 7.7 |
19.6 22.5 2.1 |
21.7 28.2 3.5 |
8.4 7.7 0.0 |
|
Olokizumab CREDO-2 (phase III)25 |
24 |
OKZ 64 mg 2 weeks + MTX (п=464) OKZ 64 mg 4 weeks + MTX (п=479) Placebo + MTX (n=243) |
74.1* 71.4 46.5 |
50.4* 50.1 22.6 |
28.7* 26.9 11.1 |
52.2*‡ 53.9 21.8 |
11.2* 12.1 4.1 |
|
Sirukumab (SIRROUND-D, phase III)57 |
16 |
SRM 100 mg 2 weeks + MTX (n=551) SRM 50 mg 4 weeks + MTX (n=553) Placebo + MTX (n=550) |
53.5* 54.8 26.4 |
33.2* 30.2 12.4 |
16.3* 14.9 3.4 |
25.5 26.0 5.6 |
8.4 7.0 3.1 |
|
Resistance to TNF-α inhibitors |
|||||||
|
Tocilizumab (RADIATE, phase III)30 |
24 |
TCZ 8 mg/kg 4 weeks + MTX (n=170) TCZ 4 mg/kg 4 weeks + MTX (n=161) Placebo + MTX (n=158) |
50.0* 30.4 10.1 |
28.8* 16.8 3.8 |
12.4* 5.0 1.3 |
30.1* 7.6 1.6 |
– |
|
Sarilumab (TARGET, phase III)36 |
24 |
SAR 200 mg 2 weeks + MTX (n=184) SAR 150 mg 2 weeks + MTX (n=181) Placebo + MTX (n=181) |
60.9* 55.8 33.7 |
40.8* 37.0 18.2 |
16.3* 19.9 7.2 |
28.8* 24.9 7.2 |
– |
|
Olokizumab (CREDO-3, phase III)37 |
12 |
OKZ 64 mg 2 weeks + MTX (п=138) OKZ 64 mg 4 weeks + MTX (п=161) Placebo + MTX (n=69) |
60.9 59.6 40.6 |
33.3 32.3 15.9 |
19.6 13.0 5.8 |
21.7 15.5 4.3 |
6.5 3.1 0.0 |
|
Sirukumab (SIRROUND-T, phase III)58 |
16 |
SRM 100 mg 2 weeks + MTX (n=292) SRM 50 mg 4 weeks + MTX (n=292) Placebo + MTX (n=294) |
42.8* 42.8 26.0 |
21.6* 20.9 8.8 |
9.9* 8.6 4.1 |
21.6 19.2 8.2 |
5.8* 3.8 3.1 |
*24 weeks; †DAS28-ESR was used in this study; ‡DAS28-CRP <3.2 was used in this study; – not provided
ACR(20/50/70) = American College of Rheumatology improvement criteria (20%/50%/70% improvement);CDAI = Clinical Disease Activity Index;DAS28-CRP = Disease Activity Score-C-reactive protein;LVM = levilimab;MTX = methotrexate;OKZ = olokizumab;SAR = sarilumab;SRM = sirukumab;TCZ = tocilizumab;weeks = duration in weeks.
Table 7: Comparative safety of interleukin 6 inhibitor therapy in rheumatoid arthritis
|
Product (trial name) |
Duration, weeks |
Safety population |
Safety, n (%) |
|||
|
≥1 AE |
Withdrawal due to AE |
SAE |
Death |
|||
|
Resistance to methotrexate |
||||||
|
Tocilizumab (OPTION, phase III)20 |
24 |
TCZ 8 mg/kg 4 weeks + МТX (n=206) TCZ 4 mg/kg 4 weeks + МТX (n=212) Placebo + МТX (n=204) |
143 (69) 151(71) 129 (63) |
12 (5.9) 14 (6.5) 6 (2.9) |
13 (6) 13 (6) 12 (6) |
0 0 0 |
|
Sarilumab (MOBILITY, phase III)22 |
52 |
SAR 200 mg 2 weeks + МТX (n=424) SAR 150 mg 2 weeks + МТX (n=431) Placebo + МТX (n=427) |
331 (78) 321 (74) 263 (61) |
59 (14) 54 (13) 20 (5) |
48 (11) 38 (9) 23 (5) |
1 (0.2) 2 (0.5) 2 (0.5) |
|
Levilimab (AURORA, phase II)56 |
12 |
LVM 162 mg + МТX1 week (n=35) LVM 162 mg + МТX2 weeks (n=35) Placebo + МТX (n=35) |
34 (97.1) 29 (82.9) 25 (71.4) |
0 4 (11.4) 2 (5.7) |
4 (11.4) 2 (5.7) 1 (2.9) |
0 2 (5.7) 0 |
|
Olokizumab CREDO-1 (phase III)24 |
24 |
OKZ 64 mg 2 weeks + МТX (п=143) OKZ 64 mg 4 weeks + МТX (п=142) Placebo + МТX (n=143) |
83 (58.0) 81 (57.0) 62 (43.7) |
7 (4.9) 5 (3.5) 1 (0.7) |
8 (5.6) 8 (5.6) 4 (2.8) |
1 (0.7) 0 (0) 0 (0) |
|
Olokizumab CREDO-2 (phase III)25 |
24 |
OKZ 64 mg 2 weeks + МТX (п=463) OKZ 64 mg 4 weeks + МТX (п=477) Placebo + МТX (n=243) |
324 (70.0) 338 (70.9) 154 (63.4) |
23 (5.0) 28 (5.8) 9 (3.7) |
22 (4.8) 20 (4.2) 12 (4.9) |
3 (0.6) 2 (0.4) 1 (0.4) |
|
Sirukumab (SIRROUND-D, phase III)57 |
16 |
SRM 100 mg 2 weeks + МТX (n=662) SRM 50 mg 4 weeks + МТX (n=663) Placebo + МТX (n=556) |
531 (80.2) 528 (79.6) 364 (65.5) |
51 (7.7) 53 (8.0) 18 (3.2) |
65 (9.8) 73 (11.0) 38 (6.8) |
3 (0.5) 7 (1.1) 1 (0.2) |
|
Resistance to TNF-α inhibitors |
||||||
|
Tocilizumab (RADIATE, phase III)30 |
24 |
TCZ 8 mg/kg 4 weeks + МТX (n=175) TCZ 4 mg/kg 4 weeks + МТX (n=163) Placebo + МТX (n=160) |
147 (84.0) 142 (87.1) 129 (80.6) |
11 (6.3) 10 (6.1) 10 (6.3) |
24 (13.7) 22 (13.5) 31 (19.4) |
0 0 0 |
|
Sarilumab (TARGET, phase III)36 |
24 |
SAR 200 mg 2 weeks + МТX (n=184) SAR 150 mg 2 weeks + МТX (n=181) Placebo + МТX (n=181) |
120 (65.2) 119 (65.7) 90 (49.7) |
17 (9.2) 18 (9.9) 8 (4.4) |
10 (5.4) 6 (3.3) 6 (3.3) |
0 0 1 (0.6) |
|
Olokizumab (CREDO-3, phase III)*37 |
24 |
OKZ 64 mg 2 weeks + МТX (п=139) OKZ 64 mg 4 weeks + МТX (п=160) Placebo + МТX (n=69) |
74 (53.2) 88 (55.0) 35 (50.7) |
6 (4.3) 9 (5.6) 2 (2.9) |
9 (6.5) 3 (1.9) 0 (0) |
0 (0) 0 (0) 0 (0) |
|
Sirukumab (SIRROUND-T, phase III)58 |
24 |
SRM 100 mg 2 weeks + МТX (n=292) SRM 50 mg 4 weeks + МТX (n=292) Placebo + МТX (n=294) |
207 (71) 194 (66) 182 (62) |
21 (7.2) 19 (6.5) 11 (3.7) |
22 (8) 28 (10) 15 (5.1) |
0 (0) 0 (0) 0 (0) |
*At week 16 all placebo-treated patients were randomized to one of the OKZ regimens.
AE = adverse effects;LVM = levilimab;MTX = methotrexate;OKZ = olokizumab;SAE = serious side effects;SAR = sarilumab;SRM = sirukumab;TCZ = tocilizumab.
Treatment considerations for the interleukin 6 inhibitors as a class
According to consensus recommendations,59,60 the products inhibiting IL-6R play a central role in the treatment of RA (level of evidence 1A); however, only IL-6 inhibitors (tocilizumab and sarilumab) are included in the current international treatment guidelines (EULAR and ACR).61,62 Further studies and real-world evidence are required to improve our knowledge about olokizumab and to clarify whether it has the same characteristics as the entire group of products inhibiting the effects of IL-6.
In patients with RA resistant to methotrexate, all bDMARDs used, including IL-6 inhibitors, TNF–α inhibitors, T –cell co-stimulation blockers (abatacept) and anti-B–cell drugs (rituximab), provide a similar efficacy.63-65 This is consistent with the data comparing olokizumab and adalimumab efficacy.25 At the same time, IL-6R inhibitors (tocilizumab and sarilumab) are more effective than TNF–α inhibitors as monotherapy in patients with contraindications to methotrexate.31,40 According to EULAR guidelines, the use of IL-6R inhibitors as drugs with a different mode of action is considered preferable to ‘switching‘ from one TNF–α inhibitor to another product in this class in patients resistant to TNF-α inhibitors;61,66 however, this provision has not yet been reflected in international recommendations on RA pharmacotherapy.61,62 It is noteworthy that combination therapy with IL-6 inhibitors (tocilizumab) and methotrexate is slightly more effective than monotherapy with IL-6R inhibitors, albeit at the cost of an increased risk of adverse events.67-70 Preliminary results indicate that the administration of JAK inhibitors sometimes allows the resistance to both TNF–α and IL-6R inhibitors to be overcome.71,72 This is probably due to the fact that the mechanism of action of JAK inhibitors is not limited to IL-6 signalling but includes other proinflammatory cytokines involved in RA immunopathogenesis.
Similarly to other antirheumatic drugs, the efficacy of IL-6 inhibitors (i.e. changes in disease activity) should be evaluated every 3 months until low disease activity is achieved (CDAI ≤10, Simple Disease Activity Index ≤11, DAS28-CRP <3.2) and every 6 months after reaching remission (ACR/EULAR criteria).61 At the same time, the evaluation of IL-6 inhibitor efficacy requires that both improvement in clinical manifestations of RA (swollen and tender joint counts) and CRP reduction are taken into account. This makes it difficult to use activity indices that include CRP (DAS28-CRP and Simple Disease Activity Index)73,74 to assess the efficacy of IL-6 inhibitor therapy. Changes in CDAI (including only swollen/tender joint counts and general assessment of the patient’s condition, without taking into account CRP level) is believed to be more informative for describing the efficacy of IL-6 inhibitors. It should be emphasized that, according to the data from CREDO 2 on CDAI, the response rate with olokizumab was similar to that with adalimumab.25 Data from 19 international registries (the JAK–pot collaboration), including 31,846 patients who received TNF–α inhibitors (17,522 courses), abatacept (2,775 courses), IL-6 inhibitors (3,863 courses) and JAK inhibitors (7,686 courses), indicate that treatment discontinuation due to lack of efficacy occurred less often with JAK inhibitors (adjusted hazard ratio 0.75) and IL-6 inhibitors (adjusted hazard ratio 0.76) compared with TNF–α inhibitors, but discontinuation due to ADR was more common (adjusted hazard ratio 1.16).75 The efficacy by the adjusted CDAI with TNF inhibitors, IL-6 inhibitors and JAK inhibitors in the groups compared did not differ but was slightly lower in the abatacept group.75
The evidence of predictive markers for the efficacy of IL-6 inhibitors in RA is limited, but they may be important for the choice of therapy. A low basal level of IL-6 is associated with the efficacy of tocilizumab or preservation of the effect after dose reduction or drug discontinuation.76,77 In contrast, a high basal level of CRP is a better predictor of IL-6 inhibitor efficacy (tocilizumab and sarilumab) compared with TNF–α inhibitors.78,79 Data concerning the relationship between body mass index and efficacy of IL-6 inhibitors are contradictory.80,81 Meta-analysis data reveal a lack of validated clinical and laboratory biomarkers for the prediction of the efficacy of IL-6 inhibitors in RA.82
The potential advantages of IL-6 inhibitors include a steroid-saving effect (glucocorticoid dose reduction or discontinuation), which is possible in two-thirds of patients.83
Safety considerations for the interleukin 6 inhibitors as a class
Contraindications to IL-6 inhibitors are already well known (i.e. hypersensitivity, severe infections and diverticulitis). Prior to prescribing treatment, a routine examination is necessary, which is also recommended when using other bDMARDs. Treatment with IL-6 inhibitors does not affect the efficacy of vaccinations protecting against pneumococcal, influenza, tetanus and, probably, severe acute respiratory coronavirus 2 (SARS-CoV-2) infections.84–87
Treatment with IL-6 inhibitors may be associated with the development of severe ADRs, including infections (sepsis). However, the incidence of infectious complications (i.e. herpes, opportunistic infections and tuberculosis, hepatitis B and C) is in the same range as with other bDMARDs.88–90 As IL-6 inhibitors can mask an infection, careful monitoring of laboratory and clinical symptoms of infections is vital during treatment with these products.
Treatment with IL-6 inhibitors (e.g. tocilizumab) does not affect or is associated with decreased frequency of malignant neoplasms compared with DMARDs, except for non-melanoma skin cancer.91,92
A gastrointestinal perforation is a specific but very rare complication of treatment with IL-6 inhibitors.93,94 Risk factors include a history of diverticulitis, old age and administration of glucocorticoids and nonsteroidal anti-inflammatory drugs.
A moderate increase in liver enzymes occurs in 50% of patients receiving IL-6 inhibitors, more commonly in combination with methotrexate. The absolute risk of severe liver damage is very low (0.04/100 patient-years).95
Inhibition of IL-6 results in dyslipoproteinaemia, specifically an increase in total cholesterol, low-density lipoproteins and triglycerides.96,97 However, this does not lead to an increased risk of cardiovascular complications or of deep vein thrombosis and pulmonary embolism, at least not compared with treatment with the TNF–α inhibitor etanercept.98 There is evidence of the important role played by IL-6 in the pathogenesis of cardiovascular pathology,99–101 and cardiovascular safety of IL-6 inhibitors has been confirmed by a number of studies.102–104 Potential positive vascular effects have been reported for tocilizumab in patients with myocardial infarction (the ASSAIL-MI trial; Clinicaltrials.gov identifier: NCT03004703)105 and for ziltivekimab, a new mAb to IL-6 in the general population of patients with atherosclerotic vascular lesions (the RESCUE trial; ClinicalTrials.gov identifier: NCT03926117).106
In patients with RA, IL-6 inhibitors have the ability to control the anaemia associated with chronic inflammation107 but are also associated with transient neutropenia and thrombocytopenia. Neutropenia does not increase the risk of infectious complications and usually does not require special treatment;108 in some cases, however, neutropenia might lead to dose adjustment or treatment discontinuation.
IL-6 inhibitors do not increase the risk of diabetes mellitus109 and may even lead to a decrease in glycated haemoglobin to a greater extent compared with TNF–α inhibitors.110,111
In patients with RA suffering from renal failure during IL-6 inhibitor therapy, there is no increase in the risk of ADRs or deterioration of renal function.112 In serial studies, IL-6 inhibitors were reported to be effective in terms of inhibiting the development and progression of renal amyloidosis,113 including in patients with RA.114
IL-6 inhibitors are believed to be relatively safe in patients with RA and interstitial lung diseases115 and demyelinating diseases.116 In addition, there are data on tocilizumab efficacy in patients with optic neuromyelitis117 and systemic sclerosis-associated interstitial lung disease,118,119 with the latter resulting in tocilizumab receiving approval by the US Food and Drug Administration for these patients.
Finally, IL-6 inhibitor therapy is associated with the stabilization of the frequency of osteoporotic fracturesn120 and exhibits a positive effect on bone metabolism.121,122
IL-6 inhibitors are very rarely associated with infusion-related reactions (about 7%) and severe hypersensitivity reactions that are not associated with the synthesis of antidrug antibodies.123,124
Interleukin 6 inhibition in coronavirus disease 2019
As IL-6 is of fundamental importance in the development of COVID-19-associated hyperinflammatory syndrome,125,126 IL-6 inhibitors are currently recommended for the treatment of this complication of SARS-CoV-2 infection in selected cases.127 Some data are available on the efficacy of olokizumab in patients with severe COVID-19;128,129 these are similar to data for tocilizumab.129
Prospects for the future development of olokizumab
Despite the long-term (>10 years) use of IL-6 inhibitors in rheumatology,13,16,130 numerous theoretical and practical issues concerning the role of these drugs in the treatment of RA require further investigation. This can be fully attributed to olokizumab, which has completed a successful phase III programme and for which real clinical practice data are beginning to accumulate. It is necessary now to implement the following tasks:
-
Investigate the differences in efficacy and safety of IL-6 inhibitors blocking IL-6R or IL-6 itself and whether the data on mAbs to IL-6R can be extrapolated to mAbs to IL-6, primarily olokizumab.
-
Develop indications for olokizumab as the first-choice bDMARD in RA in patients with inadequate response to methotrexate monotherapy.
-
Evaluate the efficacy and safety of switching from mAbs to IL-6R therapy to mAbs to IL-6 therapy (olokizumab) for medical reasons (e.g. inadequate response to therapy) and for administrative reasons.
-
Investigate the efficacy of olokizumab in patients with resistance to JAK inhibitors and vice versa.
-
Compare the efficacy and safety of olokizumab and JAK inhibitors as monotherapy and combination therapy with methotrexate and other DMARDs.
-
Investigate the efficacy and safety of olokizumab in patients with resistance to other bDMARDs (e.g. anti-B-cell therapy with rituximab, inhibition of co-stimulation of T-cells with abatacept, the effect of B-cell depletion after rituximab).
-
Describe the effect of olokizumab on the risk and course of comorbidities (i.e. cardiovascular pathology, diabetes mellitus, interstitial lung disease, osteoporotic fractures of skeletal bones, osteoarthritis) characteristic of RA131-133 and multimorbid pathologies, as well as on pain, depression, fatigue and secondary fibromyalgia syndrome,134–137 in terms of individualized RA therapy.
-
Investigate laboratory biomarkers that allow to predict efficacy and resistance in olokizumab.
-
Investigate the effect of olokizumab on the progression of joint destruction based on X-ray and magnetic resonance findings.
-
Investigate the potential to extend indications for olokizumab, taking into account the positive experience with IL-6 inhibitors (tocilizumab) for the treatment of giant cell arteritis,138-140 Takayasu arteritis,141 juvenile idiopathic arthritis,142 adult Still’s disease,143 early systemic scleroderma,118,119 autoinflammatory syndromes (familial Mediterranean fever, Behcet’s disease, TNF-associated periodic syndrome)144, hyperinflammatory syndromes, macrophage activation syndrome, haemophagocytic lymphohistiocytosis, chimeric antigen R–T-cell therapy, COVID-19 and persistent inflammation, immunosuppression and catabolism syndrome.127,145,146